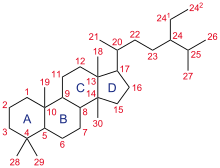
A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.
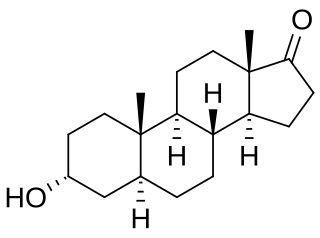
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.
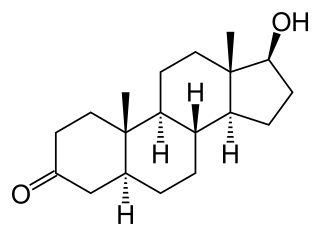
Dihydrotestosterone is an endogenous androgen sex steroid and hormone primarily involved in the growth and repair of the prostate and the penis, as well as the production of sebum and body hair composition.

5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

3-Oxo-5α-steroid 4-dehydrogenase 1 is an enzyme that in humans is encoded by the SRD5A1 gene. It is one of three forms of steroid 5α-reductase.
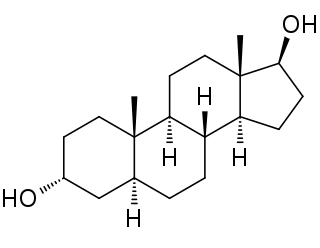
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.

Allopregnanediol, or 5α-pregnane-3α,20α-diol, is an endogenous metabolite of progesterone and allopregnanolone and an isomer of pregnanediol (5β-pregnan-3α,20α-diol). It has been found to act like a partial agonist of an allosteric site of the GABA receptor and hence might play a biological role as a neurosteroid. It has also been found to act as an agonist of the human pregnane X receptor, albeit with an EC50 that is more than an order of magnitude lower than that of other endogenous pregnanes like pregnenolone, pregnanediol, allopregnanedione, and allopregnanolone.
Pregnanolone, also known as tetrahydroprogesterone (THP), may refer to:

11β-Hydroxyprogesterone (11β-OHP), also known as 21-deoxycorticosterone, as well as 11β-hydroxypregn-4-ene-3,20-dione, is a naturally occurring, endogenous steroid and derivative of progesterone. It is a potent mineralocorticoid. Syntheses of 11β-OHP from progesterone is catalyzed by the steroid 11β-hydroxylase (CYP11B1) enzyme, and, to a lesser extent, by the aldosterone synthase enzyme (CYP11B2).

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.

The androgen backdoor pathway synthesizes physiologically relevant androgens from 21-carbon steroids (pregnanes) via 5α-reduction, bypassing testosterone. This differs from the conventional, canonical androgenic pathway, which involves testosterone.

5α-Pregnan-17α-ol-3,20-dione, also known as 17α-hydroxy-dihydroprogesterone (17‐OH-DHP) is an endogenous steroid, a metabolite of 17α-hydroxyprogesterone.

11-Ketoandrosterone is an endogenous steroid.

5α-Pregnane-3α,11β-diol-20-one, abbreviated as 3,11diOH-DHP4, also known as 3α,11β-dihydroxy-5α-pregnan-20-one, is an endogenous steroid.