
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. The dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.

A mordant or dye fixative is a substance used to set dyes on fabrics by forming a coordination complex with the dye, which then attaches to the fabric. It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. Although mordants are still used, especially by small batch dyers, it has been largely displaced in industry by directs.

Congo red is an organic compound, the sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic solvents. However, the use of Congo red has long been abandoned, primarily because of its carcinogenic properties.

Azo compounds are compounds bearing the functional group diazenyl R−N=N−R′, in which R and R′ can be either aryl or alkyl.

Sudan I, is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.
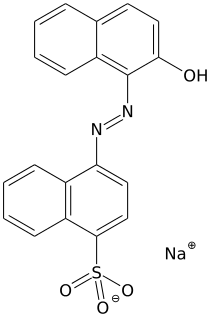
Acid dyes are anionic, soluble in water and are essentially applied from acidic bath. These dyes possess acidic groups, such as SO3H and COOH and are applied on wool, silk and nylon when ionic bond is established between protonated –NH2 group of fibre and acid group of dye. Overall wash fastness is poor although light fastness is quite good. As dye and fibre contain opposite electrical nature, strike rate and uptake of acid dye on these fibres is faster; electrolyte at higher concentration is added to retard dye uptake and to form levelled shades. Acid generates cation on fibre and temperature helps to substitute negative part of acid with anionic dye molecules.

Mordant red 19 is an organic compound with the chemical formula C16H13ClN4O5S. It is classified as an azo dye.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl. They are a commercially important family of azo compounds, i.e. compounds containing the linkage C-N=N-C. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related to azo dyes are azo pigments, which are insoluble in water and other solvents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N+
2X−
where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen.
An azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.

Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts are colorless solids. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, which smells like rotten eggs. Some commercial samples are specified as Na2S·xH2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that x is around 3. Such technical grades of sodium sulfide have a yellow appearance owing to the presence of polysulfides. These grades of sodium sulfide are marketed as 'sodium sulfide flakes'.
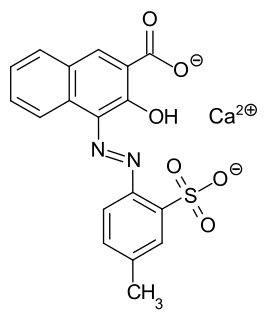
Lithol Rubine BK is a reddish synthetic azo dye. It has the appearance of a red powder and magenta when printed. It is slightly soluble in hot water, insoluble in cold water, and insoluble in ethanol. When dissolved in dimethylformamide, its absorption maximum lies at about 442 nm. It is usually supplied as a calcium salt. It is prepared by azo coupling with 3-hydroxy-2-naphthoic acid. It is used to dye plastics, paints, printing inks, and for textile printing. It is normally used as a standard magenta in the three and four color printing processes.

Sodium dichloroisocyanurate is a chemical compound widely used as a cleansing agent and disinfectant. It is a colorless, water-soluble solid. The dihydrate is also known as is the potassium salt.
Pyrazolone is 5-membered heterocycle containing 2 adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound of sodium with a dihydrogen phosphate (H2PO4−) anion. One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as mono- and dihydrates.

Sulfanilic acid is an off-white crystalline solid which finds application in quantitative analysis of nitrate and nitrite ions. The solid acid exists as a zwitterion, and has an unusually high melting point.
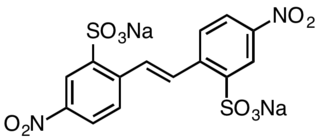
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2 This salt is a common precursor to a variety of textile dyes and optical brighteners

Synthetic dyes are found in a wide range of products such as clothes, leather accessories, and furniture. These dyes are commonly used every day. However, a side effect of their widespread use is that up to 12% of these dyes are wasted during the dying process and about 20% of this wastage enters the environment.

Tobias acid (2-amino-1-naphthalenesulfonic acid) is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, which are derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear otherwise. It is used in the synthesis of azo dyes such as C.I. Acid Yellow 19 and C.I. Pigment Red 49. It is prepared via the Bucherer reaction of 2-hydroxynaphthalene-1-sulfonic acid with ammonia and ammonium sulfite.
Basic Red 18 is a cationic azo dye used for coloring textiles. The chromophore is the cation, which contains many functional groups, but most prominently the quaternary ammonium center.



















