
An acid is a molecule or ion capable of either donating a proton (i.e., hydrogen ion, H+), known as a Brønsted–Lowry acid, or, capable of forming a covalent bond with an electron pair, known as a Lewis acid.
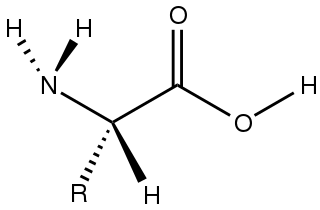
Amino acids are organic compounds that contain amino and carboxylate −CO−2 functional groups, along with a side chain specific to each amino acid. The elements present in every amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N); in addition sulfur (S) is present in the side chains of cysteine and methionine, and selenium (Se) in the less common amino acid selenocysteine. More than 500 naturally occurring amino acids are known to constitute monomer units of peptides, including proteins, as of 2020
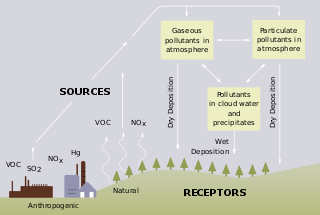
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions. Most water, including drinking water, has a neutral pH that exists between 6.5-8.5, but acid rain has a pH level lower than this and ranges from 4-5 on average. The more acidic the acid rain is, the lower its pH is. Acid rain can have harmful effects on plants, aquatic animals, and infrastructure.

A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.
Nitric acid (HNO3), also known as aqua fortis (Latin for "strong water") and spirit of niter, is a highly corrosive mineral acid.

Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin salix for willow tree. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.

Sulfuric acid (American spelling) or sulphuric acid (Commonwealth spelling), also known as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless and viscous liquid that is miscible with water.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. Usually encountered as a white solid, it is a weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

Glutamic acid is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is non-essential in humans, meaning that the body can synthesize it. It is also an excitatory neurotransmitter, in fact the most abundant one, in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons.

Boric acid, also called hydrogen borate, boracic acid, and orthoboric acid is a weak, monobasic Lewis acid of boron. However, some of its behaviour towards some chemical reactions suggest it to be tribasic acid in the Brønsted sense as well. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other chemical compounds. It has the chemical formula H3BO3 (sometimes written B(OH)3), and exists in the form of colorless crystals or a white powder that dissolves in water. When occurring as a mineral, it is called sassolite.

Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate.

Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a weak acid with the chemical formula H
3PO
4. The pure compound is a colorless solid.
ATC code B02Antihemorrhagics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup B02 is part of the anatomical group B Blood and blood forming organs.
3-Aminobenzoic acid (also known as meta-aminobenzoic acid or MABA) is an organic compound with the molecular formula H2NC6H4CO2H. MABA is a white solid, although commercial samples are often colored. It is only slightly soluble in water. It is soluble in acetone, boiling water, hot alcohol, hot chloroform and ether. It consists of a benzene ring substituted with an amino group and a carboxylic acid.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.

Hydrochloric acid (ClH3O), also known as muriatic acid, is an aqueous solution of hydrogen chloride (chemical formula: HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.

















