Related Research Articles

Haemophilia is a mostly inherited genetic disorder that impairs the body's ability to make blood clots, a process needed to stop bleeding. This results in people bleeding for a longer time after an injury, easy bruising, and an increased risk of bleeding inside joints or the brain. Those with a mild case of the disease may have symptoms only after an accident or during surgery. Bleeding into a joint can result in permanent damage while bleeding in the brain can result in long term headaches, seizures, or a decreased level of consciousness.
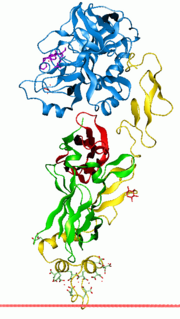
Factor VII is one of the proteins that causes blood to clot in the coagulation cascade. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa, which in turn activates factor IX and factor X.

Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.
Pegfilgrastim, sold under the brand name Neulasta among others, is a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. It serves to stimulate the production of white blood cells (neutrophils). Pegfilgrastim was developed by Amgen.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.
Defibrotide, sold under the brandname Defitelio, is a mixture of single-stranded oligonucleotides that is purified from the intestinal mucosa of pigs. It is used to treat veno-occlusive disease of the liver of people having had a bone marrow transplant, with different limitations in the US and the European Union. It works by protecting the cells lining blood vessels in the liver and preventing blood clotting; the way it does this is not well understood.

Factor VIII is a medication used to treat and prevent bleeding in people with hemophilia A and other causes of low factor VIII. Certain preparations may also be used in those with von Willebrand's disease. It is given by slow injection into a vein.

Ethyl eicosapentaenoic acid, sold under the brand name Vascepa among others, is a medication used to treat dyslipidemia and hypertriglyceridemia. It is used in combination with changes in diet in adults with hypertriglyceridemia ≥ 150 mg/dL.
Velaglucerase alfa, sold under the brand name Vpriv and manufactured by Shire plc, is a hydrolytic lysosomal glucocerebroside-specific enzyme, which is a recombinant form of glucocerebrosidase indicated as a long-term enzyme replacement therapy for those suffering of Gaucher disease Type 1. It has an identical amino acid sequence to the naturally occurring enzyme. It was approved for use by the U.S. Food and Drug Administration (FDA) on February 26, 2010.
Moroctocog alfa is a recombinant antihemophilic factor genetically engineered from Chinese hamster ovary (CHO) cell line. Chemically it is a glycoprotein. It is manufactured by Genetics Institute, Inc. and used to control and prevent hemorrhagic bleeding and prophylaxis associated with surgery or to reduce the number of spontaneous bleeding episodes in patients with hemophilia A. It is partially a recombinant coagulation factor VIII since it has an amino acid sequence which compares to the 90 + 80 kDa form of factor VIII (BDDrFVIII). It also has posttranslational modifications which are similar to those of the plasma-derived molecule. It can not prevent hemorrhagic bleeding associated with von Willebrand's disease since it is not a von Willebrand factor.

Edoxaban, sold under the brand name Lixiana among others, is an anticoagulant medication and a direct factor Xa inhibitor. It is taken by mouth.

Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is a medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.

Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests or dietary restrictions. It is taken by mouth.
Recombinant factor VIIa (rFVIIa) also known as eptacog alfa (INN) is a form of blood factor VII that has been manufactured via recombinant technology. Brand names include NovoSeven among others.
Turoctocog alfa is a recombinant antihemophilic factor VIII used for the treatment of and prophylaxis of bleeding patients with haemophilia A. It is marketed by Novo Nordisk. It was approved in the United States, the European Union, and Japan in 2013.
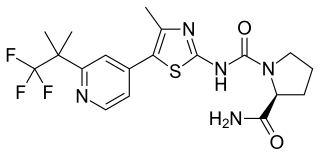
Alpelisib, sold under the brand name Piqray, is a medication sold by Novartis and used to treat certain types of breast cancer. It is used together with fulvestrant. It is taken by mouth.it is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
Emicizumab is a humanized bispecific antibody for the treatment of haemophilia A, developed by Genentech and Chugai. A Phase I clinical trial found that it was well tolerated by healthy subjects.
Vonicog alfa, sold under the brand names Vonvendi and Veyvondi, is a medicine used to control bleeding in adults with von Willebrand disease. It is a recombinant von Willebrand factor.
Efmoroctocog alfa, sold under the brand name Elocta among others, is a medication for the treatment and prophylaxis of bleeding in people with hemophilia A. Efmoroctocog alfa is a recombinant human coagulation factor VIII, Fc fusion protein (rFVIIIFc). It is produced by recombinant DNA technology in a human embryonic kidney (HEK) cell line.
Damoctocog alfa pegol, sold under the brand name Jivi is a recombinant DNA-derived, Factor VIII concentrate medication used to treat hemophilia A.
References
- ↑ "Antihemophilic factor Use During Pregnancy". Drugs.com. 20 January 2020. Retrieved 6 March 2020.
- ↑ "Obizur 500 U powder and solvent for solution for injection - Summary of Product Characteristics (SmPC)". (emc). 13 April 2017. Retrieved 3 September 2020.
- 1 2 "Obizur (antihemophilic factor- recombinant, porcine sequence kit". DailyMed. 2 January 2020. Retrieved 6 March 2020.
- 1 2 3 4 "Obizur EPAR". European Medicines Agency. 6 March 2020. Retrieved 6 March 2020.
- ↑ Burness CB, Scott LJ (May 2016). "Susoctocog Alfa: A Review in Acquired Haemophilia A". Drugs. 76 (7): 815–21. doi:10.1007/s40265-016-0576-1. PMID 27098420.
- ↑ "Obizur". U.S. Food and Drug Administration (FDA). 13 March 2018. Archived from the original on 23 April 2019. Retrieved 6 March 2020.
- ↑ "Obizur". U.S. Food and Drug Administration (FDA). 27 September 2019. STN: BL 125512. Retrieved 6 March 2020.