
The Saxifragales (saxifrages) are an order of flowering plants (Angiosperms). They are an extremely diverse group of plants which include trees, shrubs, perennial herbs, succulent and aquatic plants. The degree of diversity in terms of vegetative and floral features makes it difficult to define common features that unify the order.

Paulowniaceae are a family of flowering plants within the Lamiales. They are a monophyletic and monogeneric family of trees with currently 7 confirmed species. They were formerly placed within Scrophulariaceae sensu lato, or as a segregate of the Bignoniaceae.

Orobanchaceae, the broomrapes, is a family of mostly parasitic plants of the order Lamiales, with about 90 genera and more than 2000 species. Many of these genera were formerly included in the family Scrophulariaceae sensu lato. With its new circumscription, Orobanchaceae forms a distinct, monophyletic family. From a phylogenetic perspective, it is defined as the largest crown clade containing Orobanche major and relatives, but neither Paulownia tomentosa nor Phryma leptostachya nor Mazus japonicus.

Phrymaceae, also known as the lopseed family, is a small family of flowering plants in the order Lamiales. It has a nearly cosmopolitan distribution, but is concentrated in two centers of diversity, one in Australia, the other in western North America. Members of this family occur in diverse habitats, including deserts, river banks and mountains.

Erythranthe guttata, with the common names seep monkeyflower and common yellow monkeyflower, is a yellow bee-pollinated annual or perennial plant. It was formerly known as Mimulus guttatus.
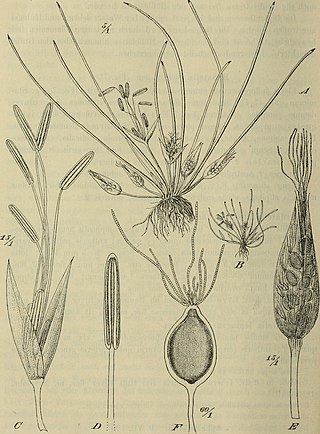
Hydatellaceae are a family of small, aquatic flowering plants. The family consists of tiny, relatively simple plants occurring in Australasia and India. It was formerly considered to be related to the grasses and sedges, but has been reassigned to the order Nymphaeales as a result of DNA and morphological analyses showing that it represents one of the earliest groups to split off in flowering-plant phylogeny, rather than having a close relationship to monocots, which it bears a superficial resemblance to due to convergent evolution. The family includes only the genus Trithuria, which has at least 13 species, although species diversity in the family has probably been substantially underestimated.

Haloragaceae is a eudicot flowering plant family in the order Saxifragales, based on the phylogenetic APG system. In the Cronquist system, it was included in the order Haloragales.

Sidalcea is a genus of the botanical family Malvaceae. It contains several species of flowering plants known generally as checkerblooms or checkermallows, or prairie mallows in the United Kingdom. They can be annuals or perennials, some rhizomatous. They are native to West and Central North America.

Opuntioideae is a subfamily of the cactus family, Cactaceae. It contains 15 genera divided into five tribes. The subfamily encompasses roughly 220–250 species, and is geographically distributed throughout the New World from Canada, to Argentina. Members of this subfamily have diverse habits, including small geophytes, hemispherical cushions, shrubs, trees, and columnar cacti consisting of indeterminate branches or determinate terete or spherical segments.

Haloragis is a genus of flowering plants in the family Haloragaceae. Some species are known commonly as seaberry and most are native to the southern hemisphere. They are annual or perennial herbs to small shrubs, and many are terrestrial wetland plants.
Semelparity and iteroparity are two contrasting reproductive strategies available to living organisms. A species is considered semelparous if it is characterized by a single reproductive episode before death, and iteroparous if it is characterized by multiple reproductive cycles over the course of its lifetime. Iteroparity can be further divided into continuous iteroparity and seasonal iteroparity Some botanists use the parallel terms monocarpy and polycarpy.

Strophostyles is monophyletic three-species genus of flowering plants in the family Fabaceae, subfamily Faboideae. Common names for the genus include wild bean and fuzzybean. It consists of annual and perennial herbaceous vines, ranging in their native distribution from Nevada, east to Florida, and north to the Great Lakes and eastern Canada. The etymology of the name is strophe (turning) + stylos (style), referring to the curve of the style within the keel petal.
Flowering synchrony is the amount of overlap between flowering periods of plants in their mating season compared to what would be expected to occur randomly under given environmental conditions. A population which is flowering synchronously has more plants flowering at the same time than would be expected to occur randomly. A population which is flowering asynchronously has fewer plants flowering at the same time than would be expected randomly. Flowering synchrony can describe synchrony of flowering periods within a year, across years, and across species in a community. There are fitness benefits and disadvantages to synchronized flowering, and it is a widespread phenomenon across pollination syndromes.

Rhinantheae is a tribe with less than 20 genera of herbaceous plants in the family Orobanchaceae.

A mixed mating system, also known as “variable inbreeding” a characteristic of many hermaphroditic seed plants, where more than one means of mating is used. Mixed mating usually refers to the production of a mixture of self-fertilized (selfed) and outbred (outcrossed) seeds. Plant mating systems influence the distribution of genetic variation within and among populations, by affecting the propensity of individuals to self-fertilize or cross-fertilize . Mixed mating systems are generally characterized by the frequency of selfing vs. outcrossing, but may include the production of asexual seeds through agamospermy. The trade offs for each strategy depend on ecological conditions, pollinator abundance and herbivory and parasite load. Mating systems are not permanent within species; they can vary with environmental factors, and through domestication when plants are bred for commercial agriculture.
Cryptic self-incompatibility (CSI) is the botanical expression that's used to describe a weakened self-incompatibility (SI) system. CSI is one expression of a mixed mating system in flowering plants. Both SI and CSI are traits that increase the frequency of fertilization of ovules by outcross pollen, as opposed to self-pollen.
Alpinia rafflesiana, commonly known in Malaysia as Tepus Telor, is a perennial herb belonging to the family Zingiberaceae. It is native to peninsular Malaysia.

Silphium integrifolium is a species of flowering plant in the family Asteraceae. Its common names include rosinweed, whole-leaf rosinweed, entire-leaf rosinweed, prairie rosinweed, and silflower. It is native to eastern North America, including Ontario in Canada and the eastern and central United States as far west as New Mexico.
Hybridization, when new offspring arise from crosses between individuals of the same or different species, results in the assemblage of diverse genetic material and can act as a stimulus for evolution. Hybrid species are often more vigorous and genetically differed than their ancestors. There are primarily two different forms of hybridization: natural hybridization in an uncontrolled environment, whereas artificial hybridization occurs primarily for the agricultural purposes.



















