
Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer and stomach cancer. It is specifically used for cancer that is HER2 receptor positive. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
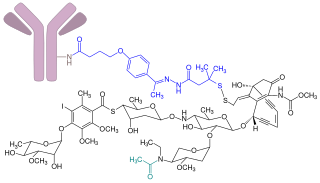
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies are used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.

Inotuzumab ozogamicin, sold under the brand name Besponsa, is an antibody-drug conjugate medication used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).

CD33 or Siglec-3 is a transmembrane receptor expressed on cells of myeloid lineage. It is usually considered myeloid-specific, but it can also be found on some lymphoid cells.
Photothermal therapy (PTT) refers to efforts to use electromagnetic radiation for the treatment of various medical conditions, including cancer. This approach is an extension of photodynamic therapy, in which a photosensitizer is excited with specific band light. This activation brings the sensitizer to an excited state where it then releases vibrational energy (heat), which is what kills the targeted cells.

Mertansine, also called DM1, is a thiol-containing maytansinoid that for therapeutic purposes is attached to a monoclonal antibody through reaction of the thiol group with a linker structure to create an antibody-drug conjugate (ADC).
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seagen in the US.

Monomethyl auristatin E (MMAE) is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is linked to a monoclonal antibody (MAB) which directs it to the cancer cells. In International Nonproprietary Names for MMAE-MAB-conjugates, the name vedotin refers to MMAE plus its linking structure to the antibody. It is a potent antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. These drugs show potency of up to 200 times that of vinblastine, another antimitotic drug used for Hodgkin lymphoma as well as other types of cancer.
Glembatumumab vedotin is an antibody-drug conjugate (ADC) that targets cancer cells expressing transmembrane glycoprotein NMB (GPNMB).

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.
The duocarmycins are members of a series of related natural products first isolated from Streptomyces bacteria in 1978. They are notable for their extreme cytotoxicity and thus represent a class of exceptionally potent antitumour antibiotics.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
ImmunoGen, Inc. is a biotechnology company focused on the development of antibody-drug conjugate (ADC) therapeutics for the treatment of cancer. ImmunoGen was founded in 1981 and is headquartered in Waltham, Massachusetts.
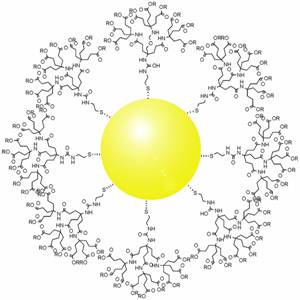
Gold nanoparticles in chemotherapy and radiotherapy is the use of colloidal gold in therapeutic treatments, often for cancer or arthritis. Gold nanoparticle technology shows promise in the advancement of cancer treatments. Some of the properties that gold nanoparticles possess, such as small size, non-toxicity and non-immunogenicity make these molecules useful candidates for targeted drug delivery systems. With tumor-targeting delivery vectors becoming smaller, the ability to by-pass the natural barriers and obstacles of the body becomes more probable. To increase specificity and likelihood of drug delivery, tumor specific ligands may be grafted onto the particles along with the chemotherapeutic drug molecules, to allow these molecules to circulate throughout the tumor without being redistributed into the body.
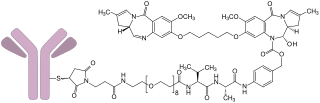
Loncastuximab tesirine, sold under the brand name Zynlonta, is a monoclonal antibody conjugate medication used to treat large B-cell lymphoma and high-grade B-cell lymphoma. It is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19.
Tisotumab vedotin, sold under the brand name Tivdak, is an antibody-drug conjugate used to treat cervical cancer. It is a combination of tisotumab, a monoclonal antibody against tissue factor, and monomethyl auristatin E (MMAE), a potent inhibitor of cell division. It is administered by infusion into a vein.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).











