
Nipple discharge is fluid from the nipple, with or without squeezing the breast. The discharge can be milky, clear, green, purulent, bloody, or faintly yellow. The consistency can be thick, thin, sticky, or watery.
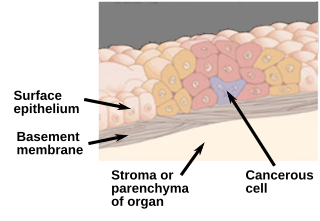
Carcinoma in situ (CIS) is a group of abnormal cells. While they are a form of neoplasm, there is disagreement over whether CIS should be classified as cancer. This controversy also depends on the exact CIS in question. Some authors do not classify them as cancer, however, recognizing that they can potentially become cancer. Others classify certain types as a non-invasive form of cancer. The term "pre-cancer" has also been used.
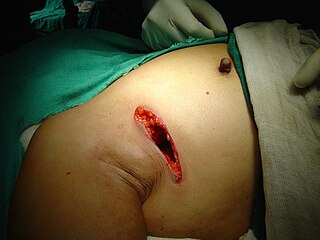
Lumpectomy is a surgical removal of a discrete portion or "lump" of breast tissue, usually in the treatment of a malignant tumor or breast cancer. It is considered a viable breast conservation therapy, as the amount of tissue removed is limited compared to a full-breast mastectomy, and thus may have physical and emotional advantages over more disfiguring treatment. Sometimes a lumpectomy may be used to either confirm or rule out that cancer has actually been detected. A lumpectomy is usually recommended to patients whose cancer has been detected early and who do not have enlarged tumors. Although a lumpectomy is used to allow for most of the breast to remain intact, the procedure may result in adverse affects that can include sensitivity and result in scar tissue, pain, and possible disfiguration of the breast if the lump taken out is significant. According to National Comprehensive Cancer Network guidelines, lumpectomy may be performed for ductal carcinoma in situ (DCIS), invasive ductal carcinoma, or other conditions.

Invasive carcinoma of no special type, invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), infiltrating ductal carcinoma (IDC) or invasive ductal carcinoma, not otherwise specified (NOS) is a disease. For international audiences this article will use "invasive carcinoma NST" because it is the preferred term of the World Health Organization (WHO).

Ductal carcinoma in situ (DCIS), also known as intraductal carcinoma, is a pre-cancerous or non-invasive cancerous lesion of the breast. DCIS is classified as Stage 0. It rarely produces symptoms or a breast lump one can feel, typically being detected through screening mammography. It has been diagnosed in a significant percentage of men.

Fibrocystic breast changes is a condition of the breasts where there may be pain, breast cysts, and breast masses. The breasts may be described as "lumpy" or "doughy". Symptoms may worsen during certain parts of the menstrual cycle due to hormonal stimulation. These are normal breast changes, not associated with cancer.
Atypical hyperplasia is a benign (noncancerous) cellular hyperplasia in which cells show some atypia. In this condition, cells look abnormal under a microscope and are increased in number.
Stereotactic biopsy, also known as stereotactic core biopsy, is a biopsy procedure that uses a computer and imaging performed in at least two planes to localize a target lesion in three-dimensional space and guide the removal of tissue for examination by a pathologist under a microscope. Stereotactic core biopsy makes use of the underlying principle of parallax to determine the depth or "Z-dimension" of the target lesion.
Comedocarcinoma is a kind of breast cancer that demonstrates comedonecrosis, which is the central necrosis of cancer cells within involved ducts. Comedocarcinomas are usually non-infiltrating and intraductal tumors, characterized as a comedo-type, high-grade ductal carcinoma in situ (DCIS). However, there have been accounts of comedocarcinoma which has then diversified into other cell types and developed into infiltrating (invasive) ductal carcinoma. Recurrence and survival rates differ for invasive breast cancer which has originated as comedocarcinoma compared with other types of cancer cells.

Lobular carcinoma in situ (LCIS) is an incidental microscopic finding with characteristic cellular morphology and multifocal tissue patterns. The condition is a laboratory diagnosis and refers to unusual cells in the lobules of the breast. The lobules and acini of the terminal duct-lobular unit (TDLU), the basic functional unit of the breast, may become distorted and undergo expansion due to the abnormal proliferation of cells comprising the structure. These changes represent a spectrum of atypical epithelial lesions that are broadly referred to as lobular neoplasia (LN).

Fat necrosis is a form of necrosis that is caused by the action of lipases on adipocytes.

High-grade prostatic intraepithelial neoplasia (HGPIN) is an abnormality of prostatic glands and believed to precede the development of prostate adenocarcinoma.
The Bethesda system (TBS), officially called The Bethesda System for Reporting Cervical Cytology, is a system for reporting cervical or vaginal cytologic diagnoses, used for reporting Pap smear results. It was introduced in 1988 and revised in 1991, 2001, and 2014. The name comes from the location of the conference, sponsored by the National Institutes of Health, that established the system.
Intraductal papillomas of the breast are benign lesions with an incidence of approximately 2-3% in humans. They result from abnormal proliferation of the epithelial cells lining the breast ducts.
A radial scar, formally radial scar of the breast, is a benign breast lesion that can radiologically mimic malignancy, i.e. cancer.

A nipple adenoma is a rare benign tumour of the breast.

A breast biopsy is usually done after a suspicious lesion is discovered on either mammography or ultrasound to get tissue for pathological diagnosis. Several methods for a breast biopsy now exist. The most appropriate method of biopsy for a patient depends upon a variety of factors, including the size, location, appearance and characteristics of the abnormality. The different types of breast biopsies include fine-needle aspiration (FNA), vacuum-assisted biopsy, core needle biopsy, and surgical excision biopsy. Breast biopsies can be done utilizing ultrasound, MRI or a stereotactic biopsy imaging guidance. Vacuum assisted biopsies are typically done using stereotactic techniques when the suspicious lesion can only be seen on mammography. On average, 5–10 biopsies of a suspicious breast lesion will lead to the diagnosis of one case of breast cancer. Needle biopsies have largely replaced open surgical biopsies in the initial assessment of imaging as well as palpable abnormalities in the breast.

Collagenous spherulosis, or simple spherulosis, is a benign finding in breast pathology. It is almost always an incidental finding, though it is occasionally associated with calcifications, which may lead to a biopsy.
Papillomatosis of the breast (PB) is a rare, benign, epitheliosis-like lesion, i.e. an overgrowth of the cells lining the ducts of glands that resembles a papilla or nipple-like nodule/tumor. PB tumors develop in the apocrine glands of the breast. PB is also termed juvenile papillomatosis because of its frequent occurrence in younger women and Swiss cheese disease because of its microscopic appearance. Rarely, PB has also been diagnosed in very young, adolescent, and adult males.
Papillary carcinomas of the breast (PCB), also termed malignant papillary carcinomas of the breast, are rare forms of the breast cancers. The World Health Organization (2019) classified papillary neoplasms of the breast into 5 types: intraductal papilloma, papillary ductal carcinoma in situ (PDCIS), encapsulated papillary carcinoma (EPC), solid-papillary carcinoma (SPC), and invasive papillary carcinoma (IPC). The latter four carcinomas are considered here; intraductal papilloma is a benign neoplasm. The World Health Organization regarded solid papillary carcinoma as having two subtypes: in situ and invasive SPC.

















