
A chordate is a deuterostomic animal belonging to the phylum Chordata. All chordates possess, at some point during their larval or adult stages, five distinctive physical characteristics (synapomorphies) that distinguish them from other taxa. These five synapomorphies are a notochord, a hollow dorsal nerve cord, an endostyle or thyroid, pharyngeal slits, and a post-anal tail. The name "chordate" comes from the first of these synapomorphies, the notochord, which plays a significant role in chordate body plan structuring and movements. Chordates are also bilaterally symmetric, have a coelom, possess a closed circulatory system, and exhibit metameric segmentation.

Hemichordata is a phylum which consists of triploblastic, enterocoelomate, and bilaterally symmetrical marine deuterostome animals, generally considered the sister group of the echinoderms. They appear in the Lower or Middle Cambrian and include two main classes: Enteropneusta, and Pterobranchia. A third class, Planctosphaeroidea, is known only from the larva of a single species, Planctosphaera pelagica. The class Graptolithina, formerly considered extinct, is now placed within the pterobranchs, represented by a single living genus Rhabdopleura.

The Lampyridae are a family of elateroid beetles with more than 2,000 described species, many of which are light-emitting. They are soft-bodied beetles commonly called fireflies, lightning bugs, or glowworms for their conspicuous production of light, mainly during twilight, to attract mates. Light production in the Lampyridae is thought to have originated as an honest warning signal that the larvae were distasteful; this was co-opted as a mating signal in the adults. In a further development, female fireflies of the genus Photuris mimic the flash pattern of Photinus species to trap their males as prey.

Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus Vibrio; in others, it is autogenic, produced by the animals themselves.

Balanoglossus is a genus of ocean-dwelling acorn worms (Enteropneusta). It has zoological importance because, being hemichordates, they are an "evolutionary link" between invertebrates and vertebrates. Balanoglossus is a deuterostome, and resembles the sea squirts (Ascidiacea) in that it possesses branchial openings, or "gill slits". It has a notochord in the upper part of the body and has no nerve chord. It does have a stomochord, however, which is a gut chord within the collar. Their heads may be as small as per 2.5 mm (1/10 in) or as large as 5 mm (1/5 in).

Pterobranchia, members of which are often called pterobranchs, is a class of small worm-shaped animals. They belong to the Hemichordata, and live in secreted tubes on the ocean floor. Pterobranchia feed by filtering plankton out of the water with the help of cilia attached to tentacles. There are about 25 known living pterobranch species in three genera, which are Rhabdopleura, Cephalodiscus, and Atubaria. On the other hand, there are several hundred extinct genera, some of which date from the Cambrian Period.
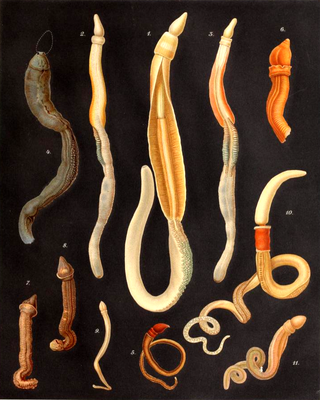
The acorn worms or Enteropneusta are a hemichordate class of invertebrates consisting of one order of the same name. The closest non-hemichordate relatives of the Enteropneusta are the echinoderms. There are 111 known species of acorn worm in the world, the main species for research being Saccoglossus kowalevskii. Two families—Harrimaniidae and Ptychoderidae—separated at least 370 million years ago.

Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose and mannose metabolism. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases, however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.

Ambulacraria, or Coelomopora, is a clade of invertebrate phyla that includes echinoderms and hemichordates; a member of this group is called an ambulacrarian. Phylogenetic analysis suggests the echinoderms and hemichordates separated around 533 million years ago. The Ambulacraria are part of the deuterostomes, a clade that also includes the many Chordata, and the few extinct species belonging to the Vetulicolia.

DNA polymerase beta, also known as POLB, is an enzyme present in eukaryotes. In humans, it is encoded by the POLB gene.

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.

Deuterostomes are bilaterian animals of the superphylum Deuterostomia, typically characterized by their anus forming before the mouth during embryonic development. Deuterostomia is further divided into 4 phyla: Chordata, Echinodermata, Hemichordata, and the extinct Vetulicolia known from Cambrian fossils. The extinct clade Cambroernida is also thought to be a member of Deuterostomia.

Saccoglossus is a genus of acorn worm. It is the largest genus in this class, with 18 species.

Harrimaniidae is a basal family of acorn worms. A taxonomic revision was undertaken in 2010, and a number of new genera and species found in the Eastern Pacific were described. In this family the development is direct without tornaria larva, and circular muscle fibers in their trunk is missing. There is some indication that Stereobalanus may be a separate basal acorn worm lineage, sister to all remaining acorn worms.

Ptychoderidae is a family of acorn worms.
Harrimania planktophilus is a marine acorn worm in the family Harrimaniidae. It lives in a burrow in sediment on the sea floor. It is only known from western Canada and was first described by Cameron in 2002. The species name is from the Greek and translates as "lover of plankton".
Torquaratoridae is a family of acorn worms (Hemichordata) that lives in deep waters between 350 and 4000 meters. They can grow up to three feet in length and have semitransparent gelatinous bodies, often brightly colored.
Spartobranchus tenuis is an extinct species of acorn worms (Enteropneusta). It existed in the Middle Cambrian. Petrified mark animals were found in British Columbia, Canada in the Burgess Shale formation. It is similar to the modern representatives of the family Harrimaniidae, distinguished by branching fiber tubes. It is a believed predecessor of Pterobranchia, but this species is intermediate between these two classes. Studies show that these tubes were lost in the line leading to modern acorn worm, but remained in the extinct graptolites. However recently Antarctic torquatorids have been found that also make tubes.

Xenambulacraria is a proposed clade of animals with bilateral symmetry as an embryo, consisting of the Xenacoelomorpha and the Ambulacraria.
Billie J. Swalla is a professor of biology at the University of Washington. She was the first female director of Friday Harbor Laboratories, where she worked from 2012 to 2019. Her lab investigates the evolution of chordates by comparative genetic and phylogenetic analysis of animal taxa.















