
A chordate is a deuterostomic animal belonging to the phylum Chordata. All chordates possess, at some point during their larval or adult stages, five distinctive physical characteristics (synapomorphies) that distinguish them from other taxa. These five synapomorphies are a notochord, a hollow dorsal nerve cord, an endostyle or thyroid, pharyngeal slits, and a post-anal tail. The name "chordate" comes from the first of these synapomorphies, the notochord, which plays a significant role in chordate body plan structuring and movements. Chordates are also bilaterally symmetric, have a coelom, possess a closed circulatory system, and exhibit metameric segmentation.

Hemichordata is a phylum which consists of triploblastic, enterocoelomate, and bilaterally symmetrical marine deuterostome animals, generally considered the sister group of the echinoderms. They appear in the Lower or Middle Cambrian and include two main classes: Enteropneusta, and Pterobranchia. A third class, Planctosphaeroidea, is known only from the larva of a single species, Planctosphaera pelagica. The class Graptolithina, formerly considered extinct, is now placed within the pterobranchs, represented by a single living genus Rhabdopleura.
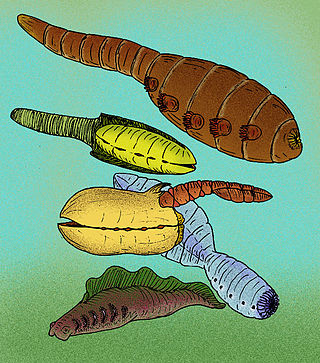
Vetulicolia is a phylum of bilaterian animals encompassing several extinct species belonging to the Cambrian period. The phylum was created by Degan Shu and his research team in 2001, and named after Vetulicola cuneata, the first species of the phylum described in 1987.

Graptolites are a group of colonial animals, members of the subclass Graptolithina within the class Pterobranchia. These filter-feeding organisms are known chiefly from fossils found from the Middle Cambrian through the Lower Carboniferous (Mississippian). A possible early graptolite, Chaunograptus, is known from the Middle Cambrian. Recent analyses have favored the idea that the living pterobranch Rhabdopleura represents an extant graptolite which diverged from the rest of the group in the Cambrian.

Pikaia gracilens is an extinct, primitive chordate animal known from the Middle Cambrian Burgess Shale of British Columbia. Described in 1911 by Charles Doolittle Walcott as an annelid, and in 1979 by Harry B. Whittington and Simon Conway Morris as a chordate, it became "the most famous early chordate fossil", or "famously known as the earliest described Cambrian chordate". It is estimated to have lived during the latter period of the Cambrian explosion. Since its initial discovery, more than a hundred specimens have been recovered.

Balanoglossus is a genus of ocean-dwelling acorn worms (Enteropneusta). It has zoological importance because, being hemichordates, they are an "evolutionary link" between invertebrates and vertebrates. Balanoglossus is a deuterostome, and resembles the sea squirts (Ascidiacea) in that it possesses branchial openings, or "gill slits". It has a notochord in the upper part of the body and has no nerve chord. It does have a stomochord, however, which is a gut chord within the collar. Their heads may be as small as per 2.5 mm (1/10 in) or as large as 5 mm (1/5 in).

Pterobranchia, members of which are often called pterobranchs, is a class of small worm-shaped animals. They belong to the Hemichordata, and live in secreted tubes on the ocean floor. Pterobranchia feed by filtering plankton out of the water with the help of cilia attached to tentacles. There are about 25 known living pterobranch species in three genera, which are Rhabdopleura, Cephalodiscus, and Atubaria. On the other hand, there are several hundred extinct genera, some of which date from the Cambrian Period.

Pharyngeal slits are filter-feeding organs found among deuterostomes. Pharyngeal slits are repeated openings that appear along the pharynx caudal to the mouth. With this position, they allow for the movement of water in the mouth and out the pharyngeal slits. It is postulated that this is how pharyngeal slits first assisted in filter-feeding, and later, with the addition of gills along their walls, aided in respiration of aquatic chordates. These repeated segments are controlled by similar developmental mechanisms. Some hemichordate species can have as many as 200 gill slits. Pharyngeal clefts resembling gill slits are transiently present during the embryonic stages of tetrapod development. The presence of pharyngeal arches and clefts in the neck of the developing human embryo famously led Ernst Haeckel to postulate that "ontogeny recapitulates phylogeny"; this hypothesis, while false, contains elements of truth, as explored by Stephen Jay Gould in Ontogeny and Phylogeny. However, it is now accepted that it is the vertebrate pharyngeal pouches and not the neck slits that are homologous to the pharyngeal slits of invertebrate chordates. Pharyngeal arches, pouches, and clefts are, at some stage of life, found in all chordates. One theory of their origin is the fusion of nephridia which opened both on the outside and the gut, creating openings between the gut and the environment.

Vetulicola is an extinct genus of marine animal discovered from the Cambrian of China. It is the eponymous member of the enigmatic phylum Vetulicolia, which is of uncertain affinities but may belong to the deuterostomes. The name was derived from Vetulicola cuneata, the first species described by Hou Xian-guang in 1987 from the Lower Cambrian Chiungchussu Formation in Chengjiang, China.

Deuterostomes are bilaterian animals of the superphylum Deuterostomia, typically characterized by their anus forming before the mouth during embryonic development. Deuterostomia is further divided into 4 phyla: Chordata, Echinodermata, Hemichordata, and the extinct Vetulicolia known from Cambrian fossils. The extinct clade Cambroernida is also thought to be a member of Deuterostomia.

Phoronids are a small phylum of marine animals that filter-feed with a lophophore, and build upright tubes of chitin to support and protect their soft bodies. They live in most of the oceans and seas, including the Arctic Ocean but excluding the Antarctic Ocean, and between the intertidal zone and about 400 meters down. Most adult phoronids are 2 cm long and about 1.5 mm wide, although the largest are 50 cm long.

Saccoglossus is a genus of acorn worm. It is the largest genus in this class, with 18 species.
In evolutionary developmental biology, inversion refers to the hypothesis that during the course of animal evolution, the structures along the dorsoventral (DV) axis have taken on an orientation opposite that of the ancestral form.

Harrimaniidae is a basal family of acorn worms. A taxonomic revision was undertaken in 2010, and a number of new genera and species found in the Eastern Pacific were described. In this family the development is direct without tornaria larva, and circular muscle fibers in their trunk is missing. There is some indication that Stereobalanus may be a separate basal acorn worm lineage, sister to all remaining acorn worms.

Ooedigera peeli is an extinct vetulicolian from the Early Cambrian of North Greenland. The front body was flattened horizontally, oval-shaped, likely bearing a reticulated or anastomosing pattern, and had 5 evenly-spaced gill pouches along the midline. The tail was also bulbous and flattened horizontally, but was divided into 7 plates connected by flexible membranes, allowing movement. Ooedigera likely swam by moving side-to-side like a fish. It may have lived in an oxygen minimum zone alongside several predators in an ecosystem based on chemosynthetic microbial mats, and was possibly a deposit or filter feeder living near the seafloor.
Harrimania planktophilus is a marine acorn worm in the family Harrimaniidae. It lives in a burrow in sediment on the sea floor. It is only known from western Canada and was first described by Cameron in 2002. The species name is from the Greek and translates as "lover of plankton".
Torquaratoridae is a family of acorn worms (Hemichordata) that lives in deep waters between 350 and 4000 meters. They can grow up to three feet in length and have semitransparent gelatinous bodies, often brightly colored.
Saccoglossus bromophenolosus is a species of acorn worm occurring in the northwestern Atlantic Ocean and the northeastern Pacific Ocean. It grows to a length of about 20 cm (8 in) and lives in a burrow in soft sediment in the intertidal and subtidal zones. The scientific name refers to 2,4-dibromophenol, a secondary metabolite present in this worm.
The Cambrian chordates are an extinct group of animals belonging to the phylum Chordata that lived during the Cambrian, between 538 and 485 million years ago. The first Cambrian chordate known is Pikaia gracilens, a lancelet-like animal from the Burgess Shale in British Columbia, Canada. The discoverer, Charles Doolittle Walcott, described it as a kind of worm (annelid) in 1911, but it was later identified as a chordate. Subsequent discoveries of other Cambrian fossils from the Burgess Shale in 1991, and from the Chengjiang biota of China in 1991, which were later found to be of chordates, several Cambrian chordates are known, with some fossils considered as putative chordates.

Balanoglossus gigas is a species of large free-living enteropneust found in the Atlantic Ocean. It is the largest acorn worm currently known, and has a strong iodoform-like odour. It is bioluminescent.




















