
A chordate is a deuterostomic animal belonging to the phylum Chordata. All chordates possess, at some point during their larval or adult stages, five distinctive physical characteristics (synapomorphies) that distinguish them from other taxa. These five synapomorphies are a notochord, a hollow dorsal nerve cord, an endostyle or thyroid, pharyngeal slits, and a post-anal tail. The name "chordate" comes from the first of these synapomorphies, the notochord, which plays a significant role in chordate body plan structuring and movements. Chordates are also bilaterally symmetric, have a coelom, possess a closed circulatory system, and exhibit metameric segmentation.

Hemichordata is a phylum which consists of triploblastic, enterocoelomate, and bilaterally symmetrical marine deuterostome animals, generally considered the sister group of the echinoderms. They appear in the Lower or Middle Cambrian and include two main classes: Enteropneusta, and Pterobranchia. A third class, Planctosphaeroidea, is known only from the larva of a single species, Planctosphaera pelagica. The class Graptolithina, formerly considered extinct, is now placed within the pterobranchs, represented by a single living genus Rhabdopleura.
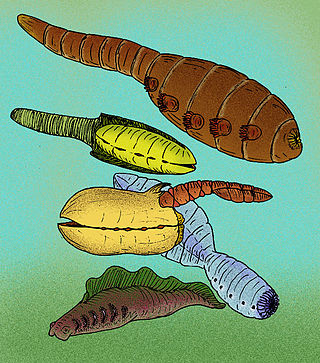
Vetulicolia is a phylum of bilaterian animals encompassing several extinct species belonging to the Cambrian period. The phylum was created by Degan Shu and his research team in 2001, and named after Vetulicola cuneata, the first species of the phylum described in 1987.

Bilateria is a large clade or infrakingdom of animals called bilaterians, characterized by bilateral symmetry during embryonic development. This means their body plans are laid around a longitudinal axis with a front and a rear end, as well as a left–right–symmetrical belly (ventral) and back (dorsal) surface. Nearly all bilaterians maintain a bilaterally symmetrical body as adults; the most notable exception is the echinoderms, which extend to pentaradial symmetry as adults, but are only bilaterally symmetrical as an embryo. Cephalization is also a characteristic feature among most bilaterians, where the special sense organs and central nerve ganglia become concentrated at the front/rostral end.

Xenoturbella is a genus of very simple bilaterians up to a few centimeters long. It contains a small number of marine benthic worm-like species.

Animals are multicellular, eukaryotic organisms in the biological kingdom Animalia. With few exceptions, animals consume organic material, breathe oxygen, have myocytes and are able to move, can reproduce sexually, and grow from a hollow sphere of cells, the blastula, during embryonic development. Animals form a clade, meaning that they arose from a single common ancestor.

Ambulacraria, or Coelomopora, is a clade of invertebrate phyla that includes echinoderms and hemichordates; a member of this group is called an ambulacrarian. Phylogenetic analysis suggests the echinoderms and hemichordates separated around 533 million years ago. The Ambulacraria are part of the deuterostomes, a clade that also includes the many Chordata, and the few extinct species belonging to the Vetulicolia.

Mitrates are an extinct group of stem group echinoderms, which may be closely related to the hemichordates. Along with the cornutes, they form one half of the Stylophora.

Protostomia is the clade of animals once thought to be characterized by the formation of the organism's mouth before its anus during embryonic development. This nature has since been discovered to be extremely variable among Protostomia's members, although the reverse is typically true of its sister clade, Deuterostomia. Well known examples of protostomes are arthropods, molluscs, annelids, flatworms and nematodes. They are also called schizocoelomates since schizocoely typically occurs in them.

The embryological origin of the mouth and anus is an important characteristic, and forms the morphological basis for separating bilaterian animals into two natural groupings: the protostomes and deuterostomes.
In evolutionary developmental biology, inversion refers to the hypothesis that during the course of animal evolution, the structures along the dorsoventral (DV) axis have taken on an orientation opposite that of the ancestral form.

Ooedigera peeli is an extinct vetulicolian from the Early Cambrian of North Greenland. The front body was flattened horizontally, oval-shaped, likely bearing a reticulated or anastomosing pattern, and had 5 evenly-spaced gill pouches along the midline. The tail was also bulbous and flattened horizontally, but was divided into 7 plates connected by flexible membranes, allowing movement. Ooedigera likely swam by moving side-to-side like a fish. It may have lived in an oxygen minimum zone alongside several predators in an ecosystem based on chemosynthetic microbial mats, and was possibly a deposit or filter feeder living near the seafloor.

Xenacoelomorpha is a small phylum of bilaterian invertebrate animals, consisting of two sister groups: xenoturbellids and acoelomorphs. This new phylum was named in February 2011 and suggested based on morphological synapomorphies, which was then confirmed by phylogenomic analyses of molecular data.

Cincta is an extinct class of echinoderms that lived only in the Middle Cambrian epoch. Homostelea is a junior synonym. The classification of cinctans is controversial, but they are probably part of the echinoderm stem group.

Saccorhytus is an extinct genus of animal possibly belonging to the superphylum Ecdysozoa, and it is represented by a single species, Saccorhytus coronarius. The organism lived approximately 540 million years ago in the beginning of the Cambrian period. Initially proposed as a deuterostome, which would make it the oldest known species of this superphylum, it has since been determined to belong to a protostome group called the ecdysozoans.

Xenoturbella bocki is a marine benthic worm-like species from the genus Xenoturbella. It is found in saltwater sea floor habitats off the coast of Europe, predominantly Sweden. It was the first species in the genus discovered. Initially it was collected by Swedish zoologist Sixten Bock in 1915, and described in 1949 by Swedish zoologist Einar Westblad. The unusual digestive structure of this species, in which a single opening is used to eat food and excrete waste, has led to considerable study and controversy as to its classification. It is a bottom-dwelling, burrowing carnivore that eats mollusks.

Xenambulacraria is a proposed clade of animals with bilateral symmetry as an embryo, consisting of the Xenacoelomorpha and the Ambulacraria.

Ctenocystoidea is an extinct clade of echinoderms, which lived during the Cambrian and Ordovician periods. Unlike other echinoderms, ctenocystoids had bilateral symmetry, or were only very slightly asymmetrical. They are believed to be one of the earliest-diverging branches of echinoderms, with their bilateral symmetry a trait shared with other deuterostomes. Ctenocystoids were once classified in the taxon Homalozoa, also known as Carpoidea, alongside cinctans, solutes, and stylophorans. Homalozoa is now recognized as a polyphyletic group of echinoderms without radial symmetry. Ctenocystoids were geographically widespread during the Middle Cambrian, with one species surviving into the Late Ordovician.

Yanjiahella biscarpa is an extinct species of Ediacaran and Early Cambrian deuterostome which may represent the earliest stem group echinoderm.

Centroneuralia is a proposed clade of animals with bilateral symmetry as an embryo, consisting of the Chordata and Protostomia, united by the presence of a central nervous system. An alternative to the traditional protostome-deuterostome dichotomy, it has found weak support in several studies. Under this hypothesis, Centroneuralia would be sister to Xenambulacraria at the base of Bilateria.























