Related Research Articles

The urea breath test is a rapid diagnostic procedure used to identify infections by Helicobacter pylori, a spiral bacterium implicated in gastritis, gastric ulcer, and peptic ulcer disease. It is based upon the ability of H. pylori to convert urea to ammonia and carbon dioxide. Urea breath tests are recommended in leading society guidelines as a preferred non-invasive choice for detecting H. pylori before and after treatment.
Peptic ulcer disease is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain, and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people with peptic ulcers have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases.
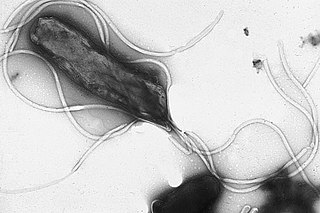
Helicobacter pylori, previously known as Campylobacter pylori, is a gram-negative, flagellated, helical bacterium. Mutants can have a rod or curved rod shape, and these are less effective. Its helical body is thought to have evolved in order to penetrate the mucous lining of the stomach, helped by its flagella, and thereby establish infection. The bacterium was first identified as the causal agent of gastric ulcers in 1983 by the Australian doctors Barry Marshall and Robin Warren.

Stomach cancer, also known as gastric cancer, is a cancer that develops from the lining of the stomach. Most cases of stomach cancers are gastric carcinomas, which can be divided into a number of subtypes, including gastric adenocarcinomas. Lymphomas and mesenchymal tumors may also develop in the stomach. Early symptoms may include heartburn, upper abdominal pain, nausea, and loss of appetite. Later signs and symptoms may include weight loss, yellowing of the skin and whites of the eyes, vomiting, difficulty swallowing, and blood in the stool, among others. The cancer may spread from the stomach to other parts of the body, particularly the liver, lungs, bones, lining of the abdomen, and lymph nodes.

Barry James Marshall is an Australian physician, Nobel Laureate in Physiology or Medicine, Professor of Clinical Microbiology and Co-Director of the Marshall Centre at the University of Western Australia. Marshall and Robin Warren showed that the bacterium Helicobacter pylori plays a major role in causing many peptic ulcers, challenging decades of medical doctrine holding that ulcers were caused primarily by stress, spicy foods, and too much acid. This discovery has allowed for a breakthrough in understanding a causative link between Helicobacter pylori infection and stomach cancer.
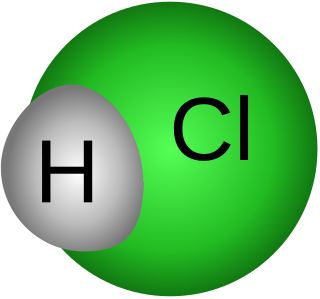
Achlorhydria and hypochlorhydria refer to states where the production of hydrochloric acid in gastric secretions of the stomach and other digestive organs is absent or low, respectively. It is associated with various other medical problems.
The pharmaceutical industry is one of the leading industries in the People's Republic of China, covering synthetic chemicals and drugs, prepared Chinese medicines, medical devices, apparatus and instruments, hygiene materials, packing materials, and pharmaceutical machinery. China has the second-largest pharmaceutical market in the world as of 2017 which is worth US$110 billion. China accounts for 20% of the world's population but only a small fraction of the global drug market. China's changing health-care environment is designed to extend basic health insurance to a larger portion of the population and give individuals greater access to products and services. Following the period of change, the pharmaceutical industry is expected to continue its expansion.

Genzyme was an American biotechnology company based in Cambridge, Massachusetts. Since its acquisition in 2011, Genzyme has been a fully owned subsidiary of Sanofi. In 2010, Genzyme was the world's third-largest biotechnology company, employing more than 11,000 people around the world. As a subsidiary of Sanofi, Genzyme has a presence in approximately 65 countries, including 17 manufacturing facilities and 9 genetic-testing laboratories. Its products are also sold in 90 countries. In 2007, Genzyme generated $3.8 billion in revenue with more than 25 products on the market. In 2006 and 2007, Genzyme was named one of Fortune magazine’s “100 Best Companies to Work for”. The company donated $83 million worth of products worldwide; in 2006, it made $11 million in cash donations. In 2005, Genzyme was awarded the National Medal of Technology, the highest level of honor awarded by the president of the United States to America's leading innovators. In February 2022, Sanofi's new corporate brand was unveiled and former entity "Sanofi Genzyme" got integrated into Sanofi.

Giaconda is an Australian biotechnology company headquartered in Sydney. The company was founded in 2004 to commercialise a number of drug combinations developed by Professor Thomas Borody, a Sydney-based gastroenterologist.

This is a timeline of the events relating to the discovery that peptic ulcer disease and some cancers are caused by H. pylori. In 2005, Barry Marshall and Robin Warren were awarded the Nobel Prize in Physiology or Medicine for their discovery that peptic ulcer disease (PUD) was primarily caused by Helicobacter pylori, a bacterium with affinity for acidic environments, such as the stomach. As a result, PUD that is associated with H. pylori is currently treated with antibiotics used to eradicate the infection. For decades prior to their discovery, it was widely believed that PUD was caused by excess acid in the stomach. During this time, acid control was the primary method of treatment for PUD, to only partial success. Among other effects, it is now known that acid suppression alters the stomach milieu to make it less amenable to H. pylori infection.
Orion Corporation, founded in 1917 and headquartered at Espoo, Finland, is a globally operating Finnish company which develops, manufactures and markets human and veterinary pharmaceuticals and active pharmaceutical ingredients for global markets. All of the company's manufacturing sites and the majority of its R&D units are in Finland.

Medac GmbH is a German, worldwide operating pharmaceutical company based in Wedel near Hamburg and Tornesch, which is privately owned.

Cancer bacteria are bacteria infectious organisms that are known or suspected to cause cancer. While cancer-associated bacteria have long been considered to be opportunistic, there is some evidence that bacteria may be directly carcinogenic. The strongest evidence to date involves the bacterium H. pylori and its role in gastric cancer.
Helicobacter pylori eradication protocols is a standard name for all treatment protocols for peptic ulcers and gastritis in the presence of Helicobacter pylori infection. The primary goal of the treatment is not only temporary relief of symptoms but also total elimination of H. pylori infection. Patients with active duodenal or gastric ulcers and those with a prior ulcer history should be tested for H. pylori. Appropriate therapy should be given for eradication. Patients with MALT lymphoma should also be tested and treated for H. pylori since eradication of this infection can induce remission in many patients when the tumor is limited to the stomach. Several consensus conferences, including the Maastricht Consensus Report, recommend testing and treating several other groups of patients but there is limited evidence of benefit. This includes patients diagnosed with gastric adenocarcinoma, patients found to have atrophic gastritis or intestinal metaplasia, as well as first-degree relatives of patients with gastric adenocarcinoma since the relatives themselves are at increased risk of gastric cancer partly due to the intrafamilial transmission of H. pylori. To date, it remains controversial whether to test and treat all patients with functional dyspepsia, gastroesophageal reflux disease, or other non-GI disorders as well as asymptomatic individuals.

Marginal zone lymphomas, also known as marginal zone B-cell lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue (MALT), the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.

Estimates place the worldwide risk of cancers from infectious causes at 16.1%. Viral infections are risk factors for cervical cancer, 80% of liver cancers, and 15–20% of the other cancers. This proportion varies in different regions of the world from a high of 32.7% in Sub-Saharan Africa to 3.3% in Australia and New Zealand.
Suominen Corporation is a Finnish company that makes nonwovens for wiping and hygiene products and healthcare applications. Suominen is the global market leader in nonwovens for wipes, and one of the world's biggest nonwovens manufacturers. Suominen's nonwovens are made into wet wipes, sanitary towels, and swabs, for example.
Exalenz Bioscience Ltd. is a biotechnology company that develops and manufactures non-invasive diagnostic medical devices for gastrointestinal and liver conditions. The company's technology uses a continuous flow of a patient's breath to diagnose illness. Exalenz is headquartered in Modi’in, Israel and is publicly traded on the Tel Aviv Stock Exchange as EXEN. U.S. headquarters are in Jersey City, New Jersey.
DiaSorin is an Italian multinational biotechnology company that produces and markets in vitro diagnostics reagent kits used in immunodiagnostics and molecular diagnostics and since July 2021, it is also active in the Life Science business. The group was founded in 2000 and is headquartered in Saluggia, Italy. Its production is at several plants located in Europe and the United States: Saluggia and Gerenzano (Italy), Dietzenbach (Germany), Stillwater, Minnesota (US), Dartford (UK). Following the acquisition of Luminex, the company acquired five additional production plants located in the United States and in Canada (Toronto). The company is a constituent of the FTSE MIB index.

A coronavirus breathalyzer is a diagnostic medical device enabling the user to test with 90% or greater accuracy the presence of severe acute respiratory syndrome coronavirus 2 in an exhaled breath. As of the first half of 2020, the idea of a practical coronavirus breathalyzer was concomitantly developed by unrelated research groups in Australia, Canada, Finland, Germany, Indonesia, Israel, Netherlands, Poland, Singapore, United Kingdom and USA.
References
- ↑ Harriet Öster (8 October 1998). "Labsystems kasvaa suuren suojassa". Tekniikka & Talous. Retrieved 2009-11-15.[ permanent dead link ]
- ↑ Osmo Suovaniemi, Helena Hentola (2004). "Aggressive Innovation and Patenting Strategy – the Way to Success and National Well-Being" (PDF). Biohit. Archived from the original (PDF) on 2011-11-10. Retrieved 2013-03-02.
- ↑ Biohit Oyj. Annual Report 2019
- ↑ Reuters. Overview of Biomet Oyj.