A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially available starting materials. Total synthesis targets can also be organometallic or inorganic. While total synthesis aims for complete construction from simple starting materials, modifying or partially synthesizing these compounds is known as semisynthesis.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.

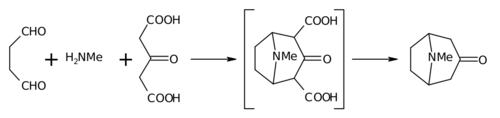
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.

The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile or elimination of an H+ ion. The outcome of the reaction depends on reaction conditions. With water and a protic acid such as sulfuric acid as the reaction medium and formaldehyde the reaction product is a 1,3-diol (3). When water is absent, the cationic intermediate loses a proton to give an allylic alcohol (4). With an excess of formaldehyde and a low reaction temperature the reaction product is a dioxane (5). When water is replaced by acetic acid the corresponding esters are formed.
Clayton Heathcock is an organic chemist, professor emeritus of chemistry, and former dean of the college of chemistry at the University of California, Berkeley. Heathcock is well known for his accomplishments in the synthesis of complex polycyclic natural products and for his contributions to the chemistry community. In 1995 he became a member of the National Academy of Sciences.
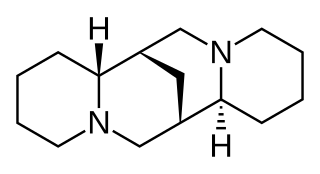
Sparteine is a class 1a antiarrhythmic agent and sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent metals calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.

Sir Alan Rushton Battersby (4 March 1925 – 10 February 2018) was an English organic chemist best known for his work to define the chemical intermediates in the biosynthetic pathway to vitamin B12 and the reaction mechanisms of the enzymes involved. His research group was also notable for its synthesis of radiolabelled precursors to study alkaloid biosynthesis and the stereochemistry of enzymic reactions. He won numerous awards including the Royal Medal in 1984 and the Copley Medal in 2000. He was knighted in the 1992 New Year Honours. Battersby died in February 2018 at the age of 92.

Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965, and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard Whitlock Jr. Professor Overman is a member of the United States National Academy of Sciences and the American Academy of Arts and Sciences. He was the recipient of the Arthur C. Cope Award in 2003, and he was awarded the Tetrahedron Prize for Creativity in Organic Chemistry for 2008.

An oxocarbeniumion is a chemical species characterized by a central sp2-hybridized carbon, an oxygen substituent, and an overall positive charge that is delocalized between the central carbon and oxygen atoms. An oxocarbenium ion is represented by two limiting resonance structures, one in the form of a carbenium ion with the positive charge on carbon and the other in the form of an oxonium species with the formal charge on oxygen. As a resonance hybrid, the true structure falls between the two. Compared to neutral carbonyl compounds like ketones or esters, the carbenium ion form is a larger contributor to the structure. They are common reactive intermediates in the hydrolysis of glycosidic bonds, and are a commonly used strategy for chemical glycosylation. These ions have since been proposed as reactive intermediates in a wide range of chemical transformations, and have been utilized in the total synthesis of several natural products. In addition, they commonly appear in mechanisms of enzyme-catalyzed biosynthesis and hydrolysis of carbohydrates in nature. Anthocyanins are natural flavylium dyes, which are stabilized oxocarbenium compounds. Anthocyanins are responsible for the colors of a wide variety of common flowers such as pansies and edible plants such as eggplant and blueberry.

The biosynthesis of cocaine has long attracted the attention of biochemists and organic chemists. This interest is partly motivated by the strong physiological effects of cocaine, but a further incentive was the unusual bicyclic structure of the molecule. The biosynthesis can be viewed as occurring in two phases, one phase leading to the N-methylpyrrolinium ring, which is preserved in the final product. The second phase incorporates a C4 unit with formation of the bicyclic tropane core.

Endiandric acid C, isolated from the tree Endiandra introrsa, is a well characterized chemical compound. Endiadric acid C is reported to have better antibiotic activity than ampicillin.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.

Anthony Gerard Martin Barrett is a British chemist, and Sir Derek Barton Professor of Synthesis, Glaxo Professor of Organic Chemistry at Imperial College London. He is Director of the Wolfson Centre for Organic Chemistry in Medical Science. He was elected a fellow of the Royal Society in 1999 and Academy of Medical Sciences in 2003. He obtained a BSc as well as PhD from Imperial College London in 1973 and 1975 respectively.

2-Carbomethoxytropinone (2-CMT) is a commonly used organic intermediate in the synthesis of cocaine and its analogues. As of at least 1999 no reaction pathway has been discovered that synthesizes cocaine-like compounds without utilizing the reduction of 2-CMT. The structure of cocaine was discovered by Richard Willstätter in 1898 after he synthesized it from 2-carbomethoxytropinone. Although it was originally believed that 2-CMT in nature was ultimately derived from ornithine and acetic acid, subsequent research has indicated other pathways exist for the biosynthesis of 2-CMT. A β-keto ester, 2-CMT exists in equilibrium with its keto–enol tautomer.

Erysodienone is a key precursor in the biosynthesis of many Erythrina-produced alkaloids. Early work was done by Derek Barton and co-workers to illustrate the biosynthetic pathways towards erythrina alkaloids. It was demonstrated that erysodienone could be synthesized from simple starting materials by a similar approach as its biosynthetic pathway, which led to the development of the biomimetic synthesis of erysodienone.

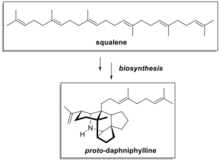
Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.