
The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere. [1] [2]

The boron cycle is the biogeochemical cycle of boron through the atmosphere, lithosphere, biosphere, and hydrosphere. [1] [2]
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
Boron in the atmosphere is derived from soil dusts, volcanic emissions, forest fires, evaporation of boric acid from seawater, biomass emissions, and sea spray. [1] Sea salt aerosols are the largest flux to the atmosphere. On land, boron cycles through the biosphere by rock weathering, and wet and dry deposition from the atmosphere. [1] [2]
The marine biosphere circulates a large reservoir of boron. Dissolved boron is delivered to the ocean by river transport, wet deposition, submarine groundwater discharge, and hydrothermal vents. [1] [2] Boron is lost from the oceans in emissions from the ocean surface, deposition of organic materials and sediments (mostly carbonates), and the subduction of ocean sediment. [1]
The boron cycle has been significantly impacted by human activity. Major anthropogenic fluxes are coal mining and combustion, oil production, emissions from industrial factories, biofuels, landfills, and mining and processing of boron ores. [1] [2] Anthropogenic boron fluxes to the hydrosphere and atmosphere have increased [1] and anthropogenic fluxes now exceed the natural boron fluxes. [1]

The carbon cycle is that part of the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycle. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. The carbon cycle comprises a sequence of events that are key to making Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration (storage) to and release from carbon sinks.

The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. In other words, it is a biologically mediated process which results in the sequestering of carbon in the deep ocean away from the atmosphere and the land. The biological pump is the biological component of the "marine carbon pump" which contains both a physical and biological component. It is the part of the broader oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).

A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cycle, the nitrogen cycle and the water cycle. In each cycle, the chemical element or molecule is transformed and cycled by living organisms and through various geological forms and reservoirs, including the atmosphere, the soil and the oceans. It can be thought of as the pathway by which a chemical substance cycles the biotic compartment and the abiotic compartments of Earth. The biotic compartment is the biosphere and the abiotic compartments are the atmosphere, lithosphere and hydrosphere.

Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).
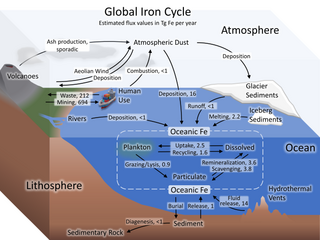
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

The mercury cycle is a biogeochemical cycle influenced by natural and anthropogenic processes that transform mercury through multiple chemical forms and environments.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Silicon is considered a bioessential element and is one of the most abundant elements on Earth. The silica cycle has significant overlap with the carbon cycle and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.

The copper cycle is the biogeochemical cycle of natural and anthropogenic exchanges of copper between reservoirs in the hydrosphere, atmosphere, biosphere, and lithosphere. Human mining and extraction activities have exerted large influence on the copper cycle.

The arsenic (As) cycle is the biogeochemical cycle of natural and anthropogenic exchanges of arsenic terms through the atmosphere, lithosphere, pedosphere, hydrosphere, and biosphere. Although arsenic is naturally abundant in the Earth's crust, long-term exposure and high concentrations of arsenic can be detrimental to human health.
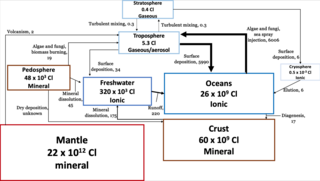
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically-produced chlorinated organics have been identified.
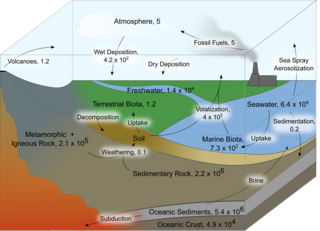
The iodine cycle is a biogeochemical cycle that primarily consists of natural and biological processes that exchange iodine through the lithosphere, hydrosphere, and atmosphere. Iodine exists in many forms, but in the environment, it generally has an oxidation state of -1, 0, or +5.

The lead cycle is the biogeochemical cycle of lead through the atmosphere, lithosphere, biosphere, and hydrosphere, which has been influenced by anthropogenic activities.

The potassium (K) cycle is the biogeochemical cycle that describes the movement of potassium throughout the Earth's lithosphere, biosphere, atmosphere, and hydrosphere.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.

The bromine cycle is a biogeochemical cycle of bromine through the atmosphere, biosphere, and hydrosphere.

The cadmium cycle is a biogeochemical cycle of dispersion and deposition of cadmium through the atmosphere, biosphere, pedosphere, and hydrosphere. Cadmium typically exists in the environment with an oxidation state of +2 but can be found with an oxidation state of +1.

The zinc cycle is a biogeochemical cycle that transports zinc through the lithosphere, hydrosphere, and biosphere.