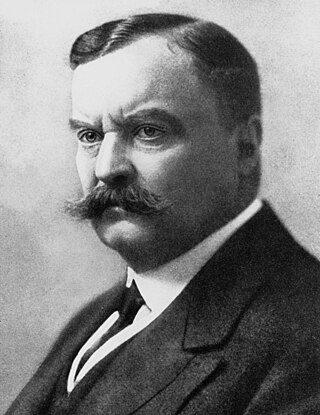Q fever or query fever is a disease caused by infection with Coxiella burnetii, a bacterium that affects humans and other animals. This organism is uncommon, but may be found in cattle, sheep, goats, and other domestic mammals, including cats and dogs. The infection results from inhalation of a spore-like small-cell variant, and from contact with the milk, urine, feces, vaginal mucus, or semen of infected animals. Rarely, the disease is tick-borne. The incubation period can range from 9 to 40 days. Humans are vulnerable to Q fever, and infection can result from even a few organisms. The bacterium is an obligate intracellular pathogenic parasite.

Orf is a farmyard pox, a type of zoonosis. It causes small pustules in the skin of primarily sheep and goats, but can also occur on the hands of humans. A pale halo forms around a red centre. It may persist for several weeks before crusting and then either resolves or leaves a hard lump. There is usually only one lesion, but there may be many, and they are not painful. Sometimes there are swollen lymph glands.
Brucellosis is a zoonosis caused by ingestion of unpasteurized milk from infected animals, or close contact with their secretions. It is also known as undulant fever, Malta fever, and Mediterranean fever.
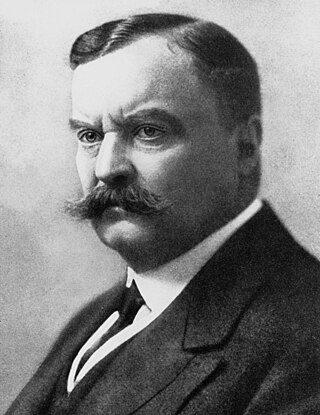
Major-General Sir David Bruce was a Scottish pathologist and microbiologist who made some of the key contributions in tropical medicine. In 1887, he discovered a bacterium, now called Brucella, that caused what was known as Malta fever. In 1894, he discovered a protozoan parasite, named Trypanosoma brucei, as the causative pathogen of nagana.

Brucella is a genus of Gram-negative bacteria, named after David Bruce (1855–1931). They are small, non-encapsulated, non-motile, facultatively intracellular coccobacilli.

Brucella suis is a bacterium that causes swine brucellosis, a zoonosis that affects pigs. The disease typically causes chronic inflammatory lesions in the reproductive organs of susceptible animals or orchitis, and may even affect joints and other organs. The most common symptom is abortion in pregnant susceptible sows at any stage of gestation. Other manifestations are temporary or permanent sterility, lameness, posterior paralysis, spondylitis, and abscess formation. It is transmitted mainly by ingestion of infected tissues or fluids, semen during breeding, and suckling infected animals.

Bernhard Lauritz Frederik Bang, was a Danish veterinarian. He discovered Brucella abortus in 1897, which came to be known as Bang's bacillus. Bang's bacillus was the cause of the contagious Bang's disease which can cause pregnant cattle to abort, and causes undulant fever in humans.

Campylobacter fetus is a rod-shaped, gram-negative species of bacteria within the genus Campylobacter of phylum Pseudomonadota. Identification of C. fetus species in infected animals or people is routinely performed by culture on blood or cefoperazone deoxycholate agar. Subspecies of C. fetus commonly causes reproductive disease in ruminants and gastrointestinal disease in humans. Transmission of C. fetus subspecies venerealis occurs mainly through venereal contact while transmission of C. fetus subspecies fetus occurs mainly through ingestion of bacteria in a contaminated environment. Infertility in cattle and abortion in sheep are common outcomes of infection associated with C. fetus subspecies venerealis and C. fetus subspecies fetus, respectively. Disease in humans occurs through zoonotic transmission of C. fetus mainly via ingestion of contaminated food or water sources. C. fetus can be diagnosed with polymerase chain reaction assays, enzyme linked immunosorbent assays and vaginal mucus agglutination testing. As vaccines are typically not efficient in preventing future spread, infected bulls are often culled. Human infections may be treated with erythromycin as antimicrobial resistance has been emerging for the fluoroquinolones.
Brucella abortus is a Gram-negative bacterium in the family Brucellaceae and is one of the causative agents of brucellosis. The rod-shaped pathogen is classified under the domain Bacteria. The prokaryotic B. abortus is non-spore-forming, non-motile and aerobic.
Professor Joseph E. Debono, a Maltese consultant physician, was appointed Demonstrator of Medicine in 1928, Professor of Materia Medica and Pharmacology in 1963 and Professor of Medicine in 1964.
Anaplasmosis is a tick-borne disease affecting ruminants, dogs, and horses, and is caused by Anaplasma bacteria. Anaplasmosis is an infectious but not contagious disease. Anaplasmosis can be transmitted through mechanical and biological vector processes. Anaplasmosis can also be referred to as "yellow bag" or "yellow fever" because the infected animal can develop a jaundiced look. Other signs of infection include weight loss, diarrhea, paleness of the skin, aggressive behavior, and high fever.

Sir Themistocles "Temi" Zammit was a Maltese archaeologist and historian, professor of chemistry, medical doctor, researcher and writer. He served as Rector (1920–26) of the Royal University of Malta and first Director of the National Museum of Archaeology in his native city, Valletta.

Alice Catherine Evans was an American microbiologist. She became a researcher at the U.S. Department of Agriculture where she investigated bacteriology in milk and cheese. She proved that Bacillus abortus caused the disease brucellosis in both cattle and humans, which led to the pasteurization of milk in the US in 1930. Evans was the first woman president elected by the Society of American Bacteriologists.
Mycoplasma ovipneumoniae is a species of Mycoplasma bacteria that most commonly inhabits and affects ovine animals, first described in 1972. M. ovipneumoniae contributes to harmful pneumonia in sheep and goats. The duration and severity of M. ovipneumoniae varies from region to region.
Brucella canis is a Gram-negative bacterium in the family Brucellaceae that causes brucellosis in dogs and other canids. It is a non-motile short-rod or coccus-shaped organism, and is oxidase, catalase, and urease positive. B. canis causes infertility in both male and female dogs. It can also cause inflammation in the eyes. The hosts of B. canis ranges from domestic animals to foxes and coyotes. It is passed from species to species via genital fluids. Treatments such as spaying, neutering, and long-term antibiotics have been used to combat B. canis. The species was first described in the United States in 1966 where mass abortions of beagles were documented. Brucella canis can be found in both pets and wild animals and lasts the lifespan of the animal it has affected. B. canis has two distinct circular chromosomes that can attribute to horizontal gene transfer.

The Maltese is a comparatively small sized rare breed of dairy goat known for its high milk production, high prolifacy and a resistance and adaptation to heat stress.
Brucella ovis is a Gram-negative coccobacillus from the Brucellaceae family. Along with Brucella melitensis, it is responsible for causing ovine brucellosis, which is a notifiable disease. B. ovis can be transmitted by the stable fly. Infection causes severe inflammation of the epididymis, particularly the tail.

Brucella ceti is a gram negative bacterial pathogen of the Brucellaceae family that causes brucellosis in cetaceans. Brucella ceti has been found in both classes of cetaceans, mysticetes and odontocetes. Brucellosis in some dolphins and porpoises can result in serious clinical signs including fetal abortions, male infertility, neurobrucellosis, cardiopathies, bone and skin lesions, stranding events, and death.

A feline zoonosis is a viral, bacterial, fungal, protozoan, nematode or arthropod infection that can be transmitted to humans from the domesticated cat, Felis catus. Some of these diseases are reemerging and newly emerging infections or infestations caused by zoonotic pathogens transmitted by cats. In some instances, the cat can display symptoms of infection and sometimes the cat remains asymptomatic. There can be serious illnesses and clinical manifestations in people who become infected. This is dependent on the immune status and age of the person. Those who live in close association with cats are more prone to these infections, but those that do not keep cats as pets can also acquire these infections as the transmission can be from cat feces and the parasites that leave their bodies.