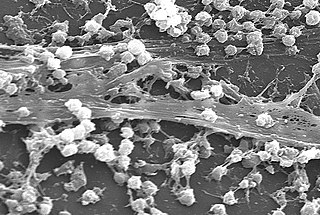
A biofilm is an syntrophic community of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric combination of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".

Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.

Burkholderia is a genus of Pseudomonadota whose pathogenic members include the Burkholderia cepacia complex, which attacks humans and Burkholderia mallei, responsible for glanders, a disease that occurs mostly in horses and related animals; Burkholderia pseudomallei, causative agent of melioidosis; and Burkholderia cepacia, an important pathogen of pulmonary infections in people with cystic fibrosis (CF). Burkholderia species is also found in marine environments. S.I. Paul et al. (2021) isolated and characterized Burkholderia cepacia from marine sponges of the Saint Martin's Island of the Bay of Bengal, Bangladesh.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics, according to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.

Burkholderia cepacia complex (BCC) is a species complex consisting of Burkholderia cepacia and at least 20 different biochemically similar species of Gram-negative bacteria. They are catalase-producing and lactose-nonfermenting. Members of BCC are opportunistic human pathogens that most often cause pneumonia in immunocompromised individuals with underlying lung disease. Patients with sickle-cell haemoglobinopathies are also at risk. The species complex also attacks young onion and tobacco plants, and displays a remarkable ability to digest oil.

Stenotrophomonas maltophilia is an aerobic, nonfermentative, Gram-negative bacterium. It is an uncommon bacterium and human infection is difficult to treat. Initially classified as Bacterium bookeri, then renamed Pseudomonas maltophilia, S. maltophilia was also grouped in the genus Xanthomonas before eventually becoming the type species of the genus Stenotrophomonas in 1993.

Burkholderia pseudomallei is a Gram-negative, bipolar, aerobic, motile rod-shaped bacterium. It is a soil-dwelling bacterium endemic in tropical and subtropical regions worldwide, particularly in Thailand and northern Australia. It was reported in 2008 that there had been an expansion of the affected regions due to significant natural disasters, and it could be found in Southern China, Hong Kong, and countries in America. B. pseudomallei, amongst other pathogens, has been found in monkeys imported into the United States from Asia for laboratory use, posing a risk that the pathogen could be introduced into the country.
Burkholderia gladioli is a species of aerobic gram-negative rod-shaped bacteria that causes disease in both humans and plants. It can also live in symbiosis with plants and fungi and is found in soil, water, the rhizosphere, and in many animals. It was formerly known as Pseudomonas marginata.

Lactonase (EC 3.1.1.81, acyl-homoserine lactonase; systematic name N-acyl-L-homoserine-lactone lactonohydrolase) is a metalloenzyme, produced by certain species of bacteria, which targets and inactivates acylated homoserine lactones (AHLs). It catalyzes the reaction
Walter Hagemeyer Burkholder was an American plant pathologist who helped establish the role of bacteria as plant pathogens. He was awarded a Ph.D. by Cornell University in 1917 and subsequently appointed as professor of plant pathology.
Pathogenomics is a field which uses high-throughput screening technology and bioinformatics to study encoded microbe resistance, as well as virulence factors (VFs), which enable a microorganism to infect a host and possibly cause disease. This includes studying genomes of pathogens which cannot be cultured outside of a host. In the past, researchers and medical professionals found it difficult to study and understand pathogenic traits of infectious organisms. With newer technology, pathogen genomes can be identified and sequenced in a much shorter time and at a lower cost, thus improving the ability to diagnose, treat, and even predict and prevent pathogenic infections and disease. It has also allowed researchers to better understand genome evolution events - gene loss, gain, duplication, rearrangement - and how those events impact pathogen resistance and ability to cause disease. This influx of information has created a need for bioinformatics tools and databases to analyze and make the vast amounts of data accessible to researchers, and it has raised ethical questions about the wisdom of reconstructing previously extinct and deadly pathogens in order to better understand virulence.
Burkholderia contaminans is a gram-negative, bacterium from the genus of Burkholderia and the family of Burkholderiaceae and belongs to the Burkholderia cepacia complex, which was isolated from cystic fibrosis patients in Argentina. Burkholderia acidipaludis can cause biliary sepsis.
Pandoraea pulmonicola is a Gram-negative, non-spore-forming, motile bacterium with a single polar flagellum, of the genus Pandoraea. P. pulmonicola has been isolated from respiratory samples of patients with cystic fibrosis and other respiratory diseases. P. pulmonicola is a part of the Burkholderia cepacia complex, which is a group of bacteria commonly associated with infections in individuals with compromised immune systems.
Staphylococcus pseudintermedius is a gram positive coccus bacteria of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well. S. pseudintermedius is an opportunistic pathogen that secretes immune modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of Staphylococcus pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.
Everett Peter Greenberg is an American microbiologist. He is the inaugural Eugene and Martha Nester Professor of Microbiology at the Department of Microbiology of the University of Washington School of Medicine. He is best known for his research on quorum sensing, and has received multiple awards for his work.
Proteobiotics are natural metabolites which are produced by fermentation process of specific probiotic strains. These small oligopeptides were originally discovered in and isolated from culture media used to grow probiotic bacteria and may account for some of the health benefits of probiotics.
Ornibactin is a siderophore, or small iron-binding compound secreted by bacteria to transport iron into the cell. Ornibactin is produced by Burkholderia cenocepacia under iron-deficient conditions. B. cenocepacia is known to opportunistically infect humans, specifically ones suffering from cystic fibrosis.
Diffusible signal factor (DSF) is found in several gram-negative bacteria and play a role in the formation of biofilms, motility, virulence, and antibiotic resistance. Xanthomonas campestris was the first bacteria known to have DSF. The synthesis of the DSF can be seen in rpfF and rpfB enzymes. An understanding of the DSF signaling mechanism could lead to further disease control.
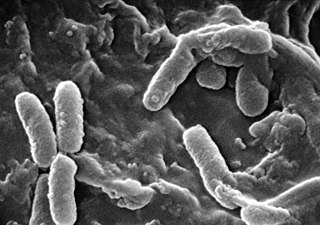
The molecule 2-heptyl-3-hydroxy-4-quinolone, also named the Pseudomonas quinolone signal (PQS), has been discovered as an intracellular link between the two major quorum sensing systems of P. aeruginosa; the las and rhl systems. These systems together control expression of virulence factors and play a major role in the formation of biofilms in Pseudomonas aeruginosa. P. aeruginosa is a gram-negative bacteria and opportunistic human pathogen that can cause serious and sometimes fatal infections in humans. Similar to other bacterial species, P. aeruginosa uses quorum sensing (QS) systems to communicate between cells in a population. This allows coordination of gene expression in a population based on changing cell densities, abundance of nutrients, and other environmental factors.










