
The term carotene (also carotin, from the Latin carota, "carrot") is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals (with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi). Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen atoms. They absorb ultraviolet, violet, and blue light and scatter orange or red light, and (in low concentrations) yellow light.

Vitamin A is a fat-soluble vitamin and an essential nutrient for animals. The term "vitamin A" encompasses a group of chemically related organic compounds that includes retinol, retinal, retinoic acid, and several provitamin (precursor) carotenoids, most notably beta-carotene. Vitamin A has multiple functions: it is essential for embryo development and growth, for maintenance of the immune system, and for vision, where it combines with the protein opsin to form rhodopsin – the light-absorbing molecule necessary for both low-light and color vision.

Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

β-Carotene (beta-carotene) is an organic, strongly coloured red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 carbons. Among the carotenes, β-carotene is distinguished by having beta-rings at both ends of the molecule. β-Carotene is biosynthesized from geranylgeranyl pyrophosphate.
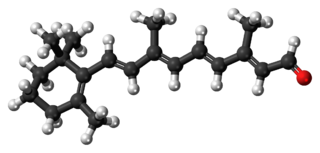
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).

Carotenoid oxygenases are a family of enzymes involved in the cleavage of carotenoids to produce, for example, retinol, commonly known as vitamin A. This family includes an enzyme known as RPE65 which is abundantly expressed in the retinal pigment epithelium where it catalyzed the formation of 11-cis-retinol from all-trans-retinyl esters.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

In enzymology, beta-carotene 15,15'-dioxygenase, (EC 1.13.11.63) is an enzyme with systematic name beta-carotene:oxygen 15,15'-dioxygenase (bond-cleaving). In human it is encoded by the BCDO2 gene. This enzyme catalyses the following chemical reaction

Dimethylaniline monooxygenase [N-oxide-forming] 1 is an enzyme that in humans is encoded by the FMO1 gene.
δ-Carotene (delta-carotene) or ε,ψ-carotene is a form of carotene with an ε-ring at one end, and the other uncyclized, labelled ψ (psi). It is an intermediate synthesis product in some photosynthetic plants between lycopene and α-carotene (β,ε-carotene) or ε-carotene (ε,ε-carotene). δ-Carotene is fat soluble. Delta-carotene contains an alpha-ionone instead of a beta-ionone ring; this conversion is carried out by the gene Del which shifts the position of the double bond in the ring structure. The formation delta-carotene under the presence of the Del gene is sensitive to high temperatures.

15-cis-phytoene desaturases, are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria. Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds. This reaction starts a biochemical pathway involving three further enzymes called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (CrtI) converts phytoene directly to lycopene by an all-trans pathway.
Carlactone synthase (EC 1.13.11.69, CCD8 (gene), MAX4 (gene), NCED8 (gene)) is an enzyme with systematic name 9-cis-10'-apo-beta-carotenal:O2 oxidoreductase (14,15-cleaving, carlactone-forming). This enzyme catalyses the following chemical reaction
Tyrosine N-monooxygenase (EC 1.14.13.41, tyrosine N-hydroxylase, CYP79A1) is an enzyme with systematic name L-tyrosine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
3-Epi-6-deoxocathasterone 23-monooxygenase (EC 1.14.13.112, cytochrome P450 90C1, CYP90D1, CYP90C1) is an enzyme with systematic name 3-epi-6-deoxocathasterone,NADPH:oxygen oxidoreductase (C-23-hydroxylating). This enzyme catalyses the following chemical reaction
Beta-carotene 3-hydroxylase (EC 1.14.13.129, beta-carotene 3,3'-monooxygenase, CrtZ) is an enzyme with systematic name beta-carotene,NADH:oxygen 3-oxidoreductase . This enzyme catalyses the following chemical reaction
Prolycopene isomerase is an enzyme with systematic name 7,9,7',9'-tetracis-lycopene cis-trans-isomerase. This enzyme catalyses the following chemical reaction
Lycopene β-cyclase is an enzyme with systematic name carotenoid beta-end group lyase (decyclizing). This enzyme catalyses the following chemical reaction

Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens.













