Related Research Articles

The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency under the Department of Health and Human Services, and is headquartered in Atlanta, Georgia.
The Mitre Corporation is an American not-for-profit organization with dual headquarters in Bedford, Massachusetts, and McLean, Virginia. It manages federally funded research and development centers (FFRDCs) supporting various U.S. government agencies in the aviation, defense, healthcare, homeland security, and cybersecurity fields, among others.
Abbott Laboratories is an American multinational medical devices and health care company with headquarters in Green Oaks, Illinois, United States. The company was founded by Chicago physician Wallace Calvin Abbott in 1888 to formulate known drugs; today, it sells medical devices, diagnostics, branded generic medicines and nutritional products. It split off its research-based pharmaceuticals business into AbbVie in 2013.

Labcorp Drug Development is a contract research organization headquartered in Burlington, North Carolina, providing nonclinical, preclinical, clinical and commercialization services to pharmaceutical and biotechnology industries. Formerly called Covance, the company is part of Labcorp, which employs more than 70,000 people worldwide.
Quest Diagnostics Incorporated is an American clinical laboratory. A Fortune 500 company, Quest operates in the United States, Puerto Rico, Mexico, and Brazil. Quest also maintains collaborative agreements with various hospitals and clinics across the globe.

JBS USA Holdings, Inc. is a meat processing company and a wholly owned subsidiary of the Brazilian multinational JBS S.A. The subsidiary was created when JBS entered the U.S. market in 2007 with its purchase of Swift & Company.

Massachusetts College of Pharmacy and Health Sciences (MCPHS) is a private university focused on health- and life-sciences education, with campuses in Boston, Massachusetts, Worcester, Massachusetts, and Manchester, New Hampshire, as well as online programs. The university provides traditional and accelerated programs of study focused on professional education in pharmacy and the health sciences.
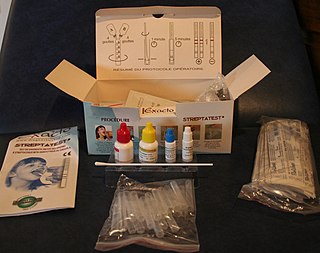
Point-of-care testing (POCT), also called near-patient testing or bedside testing, is defined as medical diagnostic testing at or near the point of care—that is, at the time and place of patient care. This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory, which entailed sending off specimens away from the point of care and then waiting hours or days to learn the results, during which time care must continue without the desired information.

A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical medical laboratories are an example of applied science, as opposed to research laboratories that focus on basic science, such as found in some academic institutions.
Laboratory Corporation of America Holdings, more commonly known as Labcorp, is an American healthcare company headquartered in Burlington, North Carolina. It operates one of the largest clinical laboratory networks in the world, with a United States network of 36 primary laboratories. Before a merger with National Health Laboratory in 1995, the company operated under the name Roche BioMedical. Labcorp performs its largest volume of specialty testing at its Center for Esoteric Testing in Burlington, North Carolina, where the company is headquartered. As of 2018, Labcorp processes 2.5 million lab tests weekly.

Randox is a Northern Irish health and toxicology company in the in vitro diagnostics industry headquartered in Crumlin, County Antrim, Northern Ireland, owned by Peter FitzGerald. The company develops diagnostic solutions for hospitals, clinical, research and molecular labs, food testing, forensic toxicology, veterinary labs and life sciences. It develops, manufactures and markets reagents and equipment for laboratory medicine, with a distribution network of 145 countries. Randox is the biggest polymerase chain reaction testing provider in the United Kingdom and Republic of Ireland. Randox received three contracts by the Department of Health and Social Care without having to compete for a tender.
Theranos Inc. was an American privately held corporation that was touted as a breakthrough health technology company. Founded in 2003 by then 19-year-old Elizabeth Holmes, Theranos raised more than US$700 million from venture capitalists and private investors, resulting in a $10 billion valuation at its peak in 2013 and 2014. The company claimed that it had devised blood tests that required very small amounts of blood and that could be performed rapidly and accurately, all using compact automated devices that the company had developed. These claims were proven to be false.
Women have been active in the cannabis industry, cannabis legalization, cannabis testing, and cannabis rights since the earliest days of commercialization, but they have also faced gendered obstacles impeding their growth in an industry worth over 12 million dollars since 2019. "The American cannabis industry accounted for $10 billion of 2018’s [global] figures, with the average U.S. dispensary pulling in $3 million a year."

This article documents the chronology and epidemiology of SARS-CoV-2 in 2019, the virus that causes coronavirus disease 2019 (COVID-19) and is responsible for the COVID-19 pandemic. The first human cases of COVID-19 known to have been identified were in Wuhan, Hubei, China, in December 2019. It marked the beginning of the 2019–2020 COVID-19 outbreak in mainland China.

Misinformation related to the COVID-19 pandemic has been propagated by various public figures, including officials of the United States government. The Trump administration in particular made a large number of misleading statements about the pandemic. A Cornell University study found that former U.S. President Donald Trump was "likely the largest driver" of the COVID-19 misinformation infodemic in English-language media, downplaying the virus and promoting unapproved drugs. Others have also been accused of spreading misinformation, including U.S. Secretary of State Mike Pompeo, backing conspiracy theories regarding the origin of the virus, U.S. senators and New York City mayor Bill de Blasio, who downplayed the virus.

Zhang Yongzhen, also known as Yong-Zhen Zhang, is a Chinese virologist known for his work relating to the COVID-19 pandemic. A professor at Fudan University, Zhang has discovered numerous RNA viruses and created a network of labs dedicated to monitoring new viruses. He led the team that sequenced and published the genome of SARS-CoV-2, the virus that causes COVID-19, in early January 2020.
LigoLab Information System is an American software company that provides software and laboratory operating systems for clinical laboratories. LigoLab develops and distributes the software tool TestDirectly, which is used for COVID-19 testing. It is based in Glendale, California.
Alexander L. Greninger is assistant director of the UW Medicine Clinical Virology Laboratory and a UW associate professor of Laboratory Medicine. His research is focused on genomic and proteomic characterization of a variety of human viruses and bacteria, with a focus on respiratory viruses and human herpesviruses.
SHIELD Illinois is the SHIELD Deployment Unit of the University of Illinois System charged with administering the covidSHIELD SARS-CoV-2 assay throughout the State of Illinois. SHIELD Illinois performed over 7.2 million SARS-CoV-2 assays during its initial program. This represents 12% of all SARS-CoV-2 tests in Illinois and more tests than 24 entire states.
References
- 1 2 "What's the Center for COVID Control? Questionable sites spotlight nation's thirst for quick testing". USA TODAY. Retrieved January 19, 2022.
dcltesting.com
- 1 2 3 "Center for COVID Control and its lab 'fraudulently reported negative test results,' Minnesota complaint says". USA TODAY. Retrieved January 19, 2022.
Minnesota Attorney General's Office
- 1 2 "COVID-19 Testing Chain Opened Pop-Ups Across The US. Now, It's Temporarily Closing Amid Federal Investigation And Mounting Complaints". Block Club Chicago. January 13, 2022. Retrieved January 19, 2022.
- ↑ "How a wedding photographer and a donut shop owner got millions in a COVID testing operation now under investigation". USA TODAY. Retrieved January 19, 2022.
- ↑ "Embattled COVID Testing Company Received $123 Million From Feds". Willamette Week. January 17, 2022. Retrieved January 19, 2022.
- 1 2 "Federal authorities investigate lab, misconduct claims tied to Center for COVID Control". USA TODAY. Retrieved January 19, 2022.
- ↑ "Covid testing company with 300 pop-up sites across U.S. faces multiple probes". NBC News. January 15, 2022. Retrieved January 19, 2022.
- ↑ "Center for COVID Control's testing sites to 'pause' as authorities in 2 states shut down centers". USA TODAY. Retrieved January 19, 2022.
- ↑ "IMPORTANT UPDATE | centerforcovidcontro". centerforcovidcontro. Archived from the original on January 20, 2022. Retrieved January 19, 2022.
Omicron Variant Surge Pressing Nation's Largest Covid-19 Testing Center Operator's Test Production, Staffing Capabilities, One Week Closure Announced
- ↑ "Attorney General Weiser, CDPHE issue demand to Center for COVID Control to stop COVID-19 testing operations in Colorado for violating state consumer protection and public health laws - Colorado Attorney General". Colorado Attorney General. January 15, 2022. Retrieved January 19, 2022.
- ↑ Worcester, City of; MA. "Cease and Desist Order Issued for COVID Testing Site on Grafton Street | City of Worcester, MA". worcesterma.gov. Retrieved January 19, 2022.
- ↑ "Oregon Department of Justice Receives 22 Additional Complaints About Embattled COVID Testing Company". Willamette Week. Retrieved January 20, 2022.