Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble in water but soluble in concentrated acids.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.
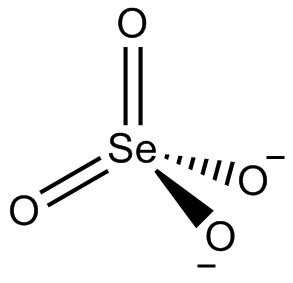
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Cerium acetate is an inorganic compound with the chemical formula of Ce(CH3COO)3. It is a white powder that is soluble in water. Its 1.5 hydrate loses water at 133°C to obtain an amorphous anhydrous form, and the amorphous phase changes to crystal at 212°C, and phase changes again at 286°C.
Praseodymium(III) selenate is an inorganic compound, the salt of praseodymium and selenic acid with the chemical formula Pr2(SeO4)3. It forms green crystals when hydrated.

Cerium monoselenide is an inorganic compound with the chemical formula CeSe. It exists in the form of Ce3+Se2−(e−).

Thulium(III) selenate is an inorganic compound, with the chemical formula Tm2(SeO4)3. It can be obtained by reacting a thulium(III) oxide and selenic acid solution and crystallizing it. It crystallises with ammonium selenate in an aqueous solution to obtain NH4Tm(SeO4)2·3H2O.

Erbium(III) selenate is an inorganic compound, with the chemical formula Er2(SeO4)3. It exists as an anhydrate or an octahydrate.

Holmium(III) selenate is an inorganic compound with the chemical formula Ho2(SeO4)3. It exists in the anhydrous form and as an octahydrate. It can be obtained by dissolving holmium(III) oxide in selenic acid solution and evaporating and crystallizing it. It co-crystallizes with other selenates in solution to obtain complex salts such as K3Ho(SeO4)3·nH2O, NH4Ho(SeO4)2·3H2O and CH3NH3Ho(SeO4)2·5H2O.
Cerium(IV) selenate is an inorganic compound with the chemical formula Ce(SeO4)2.
Zirconium selenate is an inorganic compound with the chemical formula Zr(SeO4)2. Its tetrahydrate can be obtained by the reaction of selenic acid and a saturated aqueous solution of zirconium oxychloride octahydrate (or zirconium hydroxide). The tetrahydrate belongs to the orthorhombic crystal system and is isostructural with Zr(SO4)2·4H2O. It loses water when heated and becomes anhydrous at 580 °C. It reacts with potassium fluoride to obtain K2Zr(SeO4)2F2·3H2O.









