Related Research Articles
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.

In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer chemistry, an addition polymer is a polymer that forms by simple linking of monomers without the co-generation of other products. Addition polymerization differs from condensation polymerization, which does co-generate a product, usually water. Addition polymers can be formed by chain polymerization, when the polymer is formed by the sequential addition of monomer units to an active site in a chain reaction, or by polyaddition, when the polymer is formed by addition reactions between species of all degrees of polymerization. Addition polymers are formed by the addition of some simple monomer units repeatedly. Generally polymers are unsaturated compounds like alkenes, alkalines etc. The addition polymerization mainly takes place in free radical mechanism. The free radical mechanism of addition polymerization completed by three steps i.e. Initiation of free radical, Chain propagation, Termination of chain.
Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
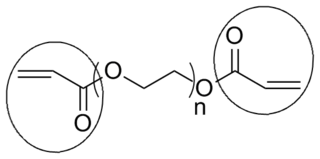
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, they are functional groups that are at the very ends of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and raman spectroscopy. These groups are important for the analysis of polymers and for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
In polymer chemistry, anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent (CTA) in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed under conditions that favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
Chain propagation (sometimes referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a chemical chain reaction. For example, in the chlorination of methane, there is a two-step propagation cycle involving as chain carriers a chlorine atom and a methyl radical which are regenerated alternately:
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.
In polymer chemistry the kinetic chain length of a polymer, ν, is the average number of units called monomers added to a growing chain during chain-growth polymerization. During this process, a polymer chain is formed when monomers are bonded together to form long chains known as polymers. Kinetic chain length is defined as the average number of monomers that react with an active center such as a radical from initiation to termination.
Cobalt based catalysts, when used in radical polymerization, have several main advantages especially in slowing down the reaction rate, allowing for the synthesis of polymers with peculiar properties. As starting the reaction does need a real radical initiator, the cobalt species is not the only used catalyst, it is a mediator. For this reason this type of reaction is referred to as cobalt mediated radical polymerization.
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
In chemistry, cationic polymerization is a type of chain growth polymerization in which a cationic initiator transfers charge to a monomer which then becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer. The types of monomers necessary for cationic polymerization are limited to alkenes with electron-donating substituents and heterocycles. Similar to anionic polymerization reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain. Cationic polymerization is used in the production of polyisobutylene and poly(N-vinylcarbazole) (PVK).
Radical disproportionation encompasses a group of reactions in organic chemistry in which two radicals react to form two different non-radical products. Radicals in chemistry are defined as reactive atoms or molecules that contain an unpaired electron or electrons in an open shell. The unpaired electrons can cause radicals to be unstable and reactive. Reactions in radical chemistry can generate both radical and non-radical products. Radical disproportionation reactions can occur with many radicals in solution and in the gas phase. Due to the reactive nature of radical molecules, disproportionation proceeds rapidly and requires little to no activation energy. The most thoroughly studied radical disproportionation reactions have been conducted with alkyl radicals, but there are many organic molecules that can exhibit more complex, multi-step disproportionation reactions.
Reversible deactivation radical polymerizations (RDRPs) are members of the class of reversible deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without chain transfer or chain termination reactions. Several different names have been used in literature, which are:
Ionic polymerization is a chain-growth polymerization in which active centers are ions or ion pairs. It can be considered as an alternative to radical polymerization, and may refer to anionic polymerization or cationic polymerization.
In polymer chemistry, degenerative chain transfer is a process that can occur in a radical polymerization whereby reactivity of active centres are changed, hence significantly influencing the molecular weight distribution of the resulting product.
References
- ↑ Jenkins, Aubrey D.; Jones, Richard G.; Moad, Graeme (2010). "Terminology for reversible-deactivation radical polymerization previously called "controlled" radical or "living" radical polymerization (IUPAC Recommendations 2010)". Pure and Applied Chemistry . 82 (2): 483–491. doi: 10.1351/PAC-REP-08-04-03 . S2CID 98243937.
- 1 2 3 Harry R. Allcock and Frederick W. Lampe Contemporary Polymer Chemistry (3rd ed., Prentice Hall 2003), p.70-72 ISBN 0-13-065056-0