Related Research Articles

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees.

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled.
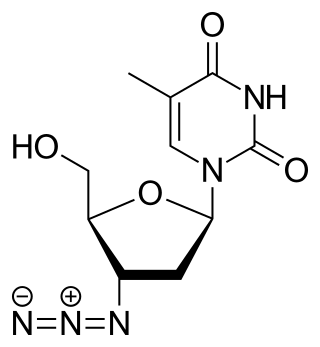
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.

Aciclovir (ACV), also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.
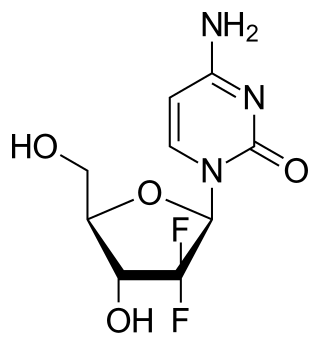
Gemcitabine, with brand names including Gemzar, is a chemotherapy medication. It treats cancers including testicular cancer, breast cancer, ovarian cancer, non-small cell lung cancer, pancreatic cancer, and bladder cancer. It is administered by intravenous infusion. It acts against neoplastic growth, and it inhibits the replication of Orthohepevirus A, the causative agent of Hepatitis E, through upregulation of interferon signaling.

Azacitidine, sold under the brand name Vidaza among others, is used for the treatment of myelodysplastic syndrome, myeloid leukemia, and juvenile myelomonocytic leukemia. It is a chemical analog of cytidine, a nucleoside in DNA and RNA. Azacitidine and its deoxy derivative, decitabine were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.

Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates.
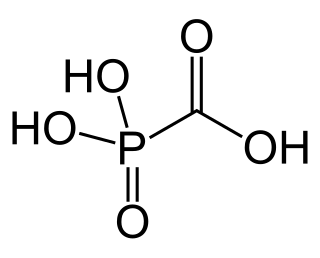
Foscarnet (phosphonomethanoic acid), known by its brand name Foscavir, is an antiviral medication which is primarily used to treat viral infections involving the Herpesviridae family. It is classified as a pyrophosphate analog DNA polymerase inhibitor. Foscarnet is the conjugate base of a chemical compound with the formula HO2CPO3H2 (Trisodium phosphonoformate).

Rudi Pauwels is a Belgian pharmacologist and biotech entrepreneur.

Telbivudine is an antiviral drug used in the treatment of hepatitis B infection. It is marketed by Swiss pharmaceutical company Novartis under the trade names Sebivo and Tyzeka. Clinical trials have shown it to be significantly more effective than lamivudine or adefovir, and less likely to cause resistance. However, HBV signature resistance mutation M204I or L180M+M204V have been associated with Telbivudine resistance.

Aplaviroc is a CCR5 entry inhibitor that belongs to a class of 2,5-diketopiperazines developed for the treatment of HIV infection. It was developed by GlaxoSmithKline.

Dexelvucitabine is a failed experimental agent for the management of human immunodeficiency virus infection. It is a cytidine nucleoside analog and nucleoside reverse transcriptase inhibitor. that inhibits HIV-1 replication in vitro. During phase II clinical trials there was some indication of a decreased mean viral load in patients with infected human immunodeficiency virus.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.
Deborah Persaud is a Guyanese-born American virologist who primarily works on HIV/AIDS at Johns Hopkins Children's Center.
Margaret Tisdale was a Welsh-born clinical virologist known for her studies of antiviral resistance in HIV and influenza virus, and for coordinating the development of the anti-influenza drug zanamivir.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
Bictegravir/emtricitabine/tenofovir alafenamide, sold under the brand name Biktarvy, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. One tablet, taken orally once daily, contains 50 mg bictegravir, 200 mg emtricitabine, and 25 mg tenofovir alafenamide. It was approved for use in the United States in February 2018, and for use in the European Union in June 2018.
Katherine Seley-Radtke is an American medicinal chemist who specializes in the discovery and design of novel nucleoside or nucleotide based enzyme inhibitors that may be used to treat infections or cancer. She has authored over 90 peer-reviewed publications,is an inventor of five issued US patents, and is a Professor in the Department of Chemistry & Biochemistry at the University of Maryland, Baltimore County. Her international impact includes scientific collaborations, policy advising and diplomatic appointments in biosecurity efforts.
References
- ↑ "Christine L Clouser, PhD".
- ↑ "Volume 70 Issue 4 | Review of the journal Biology of Reproduction".
- ↑ "Drug design success propels efforts to fight HIV with a combination of 2 FDA-approved drugs". ScienceDaily. Retrieved April 25, 2015.
- ↑ Clouser, Christine; Crankshaw, Duane; Briggs, Jacquie; Mansky, Louis; Patterson, Steven; Mullett, Mary; O'Sullivan, Gerard; Holtz, Colleen (April 2012). "Activity of a Novel Combined Antiretroviral Therapy of Gemcitabine and Decitabine in a Mouse Model for HIV-1". Antimicrobial Agents and Chemotherapy. 56 (4): 1942–1948. doi:10.1128/AAC.06161-11. PMC 3318345 . PMID 22271861.
- ↑ Mansky, Louis; Clouser, Christine; Bonnac, Laurent; Patterson, Steven (December 2014). "Characterization of Permeability, Stability and Anti-HIV-1 Activity of Decitabine and Gemcitabine Divalerate Prodrugs". Antiviral Chemistry & Chemotherapy. 23 (6): 223–230. doi:10.3851/IMP2682. PMC 4489529 . PMID 23994876.
- ↑ Clouser, Christine; Mansky, Louis; Patterson, Steven (July 2010). "Exploiting Drug Repositioning for Discovery of a Novel HIV Combination Therapy". Journal of Virology. 84 (18): 9301–9308. doi:10.1128/JVI.01006-10. PMC 2937626 . PMID 20610712.
- ↑ Dapp, Michael; Clouser, Christine; Mansky, Louis; Patterson, Steven (September 2009). "5-Azacytidine Can Induce Lethal Mutagenesis in Human Immunodeficiency Virus Type 1". Journal of Virology. 83 (22): 11950–11958. doi:10.1128/JVI.01406-09. PMC 2772699 . PMID 19726509.