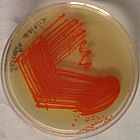
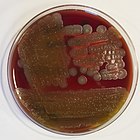
Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

An agar plate is a Petri dish that contains a growth medium solidified with agar, used to culture microorganisms. Sometimes selective compounds are added to influence growth, such as antibiotics.

Methicillin, also known as meticillin, is a narrow-spectrum β-lactam antibiotic of the penicillin class.

A microbiological culture, or microbial culture, is a method of multiplying microbial organisms by letting them reproduce in predetermined culture medium under controlled laboratory conditions. Microbial cultures are foundational and basic diagnostic methods used as a research tool in molecular biology.

A coccus is any bacterium or archaeon that has a spherical, ovoid, or generally round shape. Bacteria are categorized based on their shapes into three classes: cocci (spherical-shaped), bacillus (rod-shaped) and spirochetes (spiral-shaped) cells. Coccus refers to the shape of the bacteria, and can contain multiple genera, such as staphylococci or streptococci. Cocci can grow in pairs, chains, or clusters, depending on their orientation and attachment during cell division. In contrast to many bacilli-shaped bacteria, most cocci bacteria do not have flagella and are non-motile.

The viridans streptococci are a large group of commensal streptococcal Gram-positive bacteria species that are α-hemolytic, producing a green coloration on blood agar plates, although some species in this group are actually γ-hemolytic, meaning they produce no change on blood agar. The pseudo-taxonomic term "Streptococcus viridans" is often used to refer to this group of species, but writers who do not like to use the pseudotaxonomic term prefer the terms viridans streptococci, viridans group streptococci (VGS), or viridans streptococcal species.

A blood culture is a medical laboratory test used to detect bacteria or fungi in a person's blood. Under normal conditions, the blood does not contain microorganisms: their presence can indicate a bloodstream infection such as bacteremia or fungemia, which in severe cases may result in sepsis. By culturing the blood, microbes can be identified and tested for resistance to antimicrobial drugs, which allows clinicians to provide an effective treatment.

A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss Physcomitrella patens. Different types of media are used for growing different types of cells.

Hemolysis is the breakdown of red blood cells. The ability of bacterial colonies to induce hemolysis when grown on blood agar is used to classify certain microorganisms. This is particularly useful in classifying streptococcal species. A substance that causes hemolysis is a hemolysin.
A colony-forming unit is a unit used in microbiology. It estimates the number of bacteria or fungal cells in a sample which are viable, able to multiply via binary fission under the controlled conditions. Counting with colony-forming units requires culturing the microbes and counts only viable cells, in contrast with microscopic examination which counts all cells, living or dead. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty.
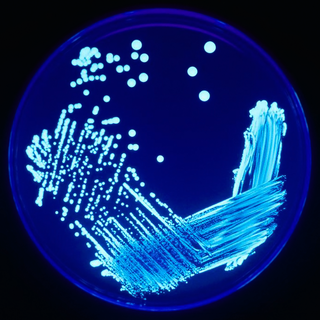
In microbiology, streaking is a technique used to isolate a pure strain from a single species of microorganism, often bacteria. Samples can then be taken from the resulting colonies and a microbiological culture can be grown on a new plate so that the organism can be identified, studied, or tested.

Mannitol salt agar or MSA is a commonly used selective and differential growth medium in microbiology. It encourages the growth of a group of certain bacteria while inhibiting the growth of others. . It contains a high concentration of salt (NaCl) which is inhibitory to most bacteria - making MSA selective against most Gram-negative and selective for some Gram-positive bacteria that tolerate high salt concentrations. It is also a differential medium for mannitol-fermenting staphylococci, containing carbohydrate mannitol and the indicator phenol red, a pH indicator for detecting acid produced by mannitol-fermenting staphylococci. Staphylococcus aureus produces yellow colonies with yellow zones, whereas other coagulase-negative staphylococci produce small pink or red colonies with no colour change to the medium. If an organism can ferment mannitol, an acidic byproduct is formed that causes the phenol red in the agar to turn yellow. It is used for the selective isolation of presumptive pathogenic (pp) Staphylococcus species.

The 3M Petrifilm plate is an all-in-one plating system made by the Food Safety Division of the 3M Company. They are heavily used in many microbiology-related industries and fields to culture various micro-organisms and are meant to be a more efficient method for detection and enumeration compared to conventional plating techniques. A majority of its use is for the testing of foodstuffs.

Etest is a way of determining antimicrobial sensitivity by placing a strip impregnated with antimicrobials onto an agar plate. A strain of bacterium or fungus will not grow near a concentration of antibiotic or antifungal if it is sensitive. For some microbial and antimicrobial combinations, the results can be used to determine a minimum inhibitory concentration (MIC). Etest is a proprietary system manufactured by bioMérieux. It is a laboratory test used in healthcare settings to help guide physicians by indicating what concentration of antimicrobial could successfully be used to treat patients' infections.
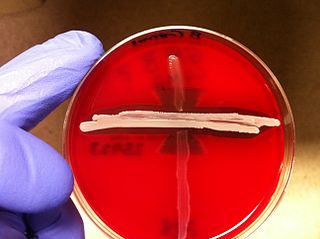
The CAMP test (Christie–Atkins–Munch-Peterson) is a test to identify group B β-hemolytic streptococci based on their formation of a substance that enlarges the area of hemolysis formed by the β-hemolysin elaborated from Staphylococcus aureus.
Staphylococcus schleiferi is a Gram-positive, cocci-shaped bacterium of the family Staphylococcaceae. It is facultatively anaerobic, coagulase-variable, and can be readily cultured on blood agar where the bacterium tends to form opaque, non-pigmented colonies and beta (β) hemolysis. There exists two subspecies under the species S. schleiferi: Staphylococcus schleiferi subsp. schleiferi and Staphylococcus schleiferi subsp. coagulans.
Staphylococcus pseudintermedius is a gram positive coccus bacteria of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well.S. pseudintermedius is an opportunistic pathogen that secretes immune modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of Staphylococcus pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.

Granada medium is a selective and differential culture medium designed to selectively isolate Streptococcus agalactiae and differentiate it from other microorganisms. Granada Medium was developed by Dr. Manuel Rosa-Fraile et al. at the Service of Microbiology in the Hospital Virgen de las Nieves in Granada (Spain).
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil flora, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.
Diagnostic microbiology is the study of microbial identification. Since the discovery of the germ theory of disease, scientists have been finding ways to harvest specific organisms. Using methods such as differential media or genome sequencing, physicians and scientists can observe novel functions in organisms for more effective and accurate diagnosis of organisms. Methods used in diagnostic microbiology are often used to take advantage of a particular difference in organisms and attain information about what species it can be identified as, which is often through a reference of previous studies. New studies provide information that others can reference so that scientists can attain a basic understanding of the organism they are examining.