
Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Sporothrix schenckii, a fungus that can be found worldwide in the environment, is named for medical student Benjamin Schenck, who in 1896 was the first to isolate it from a human specimen. The species is present in soil as well as in and on living and decomposing plant material such as peat moss. It can infect humans as well as animals and is the causative agent of sporotrichosis, commonly known as "rose handler's disease." The most common route of infection is the introduction of spores to the body through a cut or puncture wound in the skin. Infection commonly occurs in otherwise healthy individuals but is rarely life-threatening and can be treated with antifungals. In the environment it is found growing as filamentous hyphae. In host tissue it is found as a yeast. The transition between the hyphal and yeast forms is temperature dependent making S. schenckii a thermally dimorphic fungus.

Cochliobolus lunatus is a fungal plant pathogen that can cause disease in humans and other animals. The anamorph of this fungus is known as Curvularia lunata, while C. lunatus denotes the teleomorph or sexual stage. They are, however, the same biological entity. C. lunatus is the most commonly reported species in clinical cases of reported Cochliobolus infection.

Setosphaeria rostrata is a heat tolerant fungus with an asexual reproductive form (anamorph) known as Exserohilum rostratum. This fungus is a common plant pathogen, causing leaf spots as well as crown rot and root rot in grasses. It is also found in soils and on textiles in subtropical and tropical regions. Exserohilum rostratum is one of the 35 Exserohilum species implicated uncommonly as opportunistic pathogens of humans where it is an etiologic agent of sinusitis, keratitis, skin lesions and an often fatal meningoencephalitis. Infections caused by this species are most often seen in regions with hot climates like Israel, India and the southern USA.
Acremonium strictum is an environmentally widespread saprotroph species found in soil, plant debris, and rotting mushrooms. Isolates have been collected in North and Central America, Asia, Europe and Egypt. A. strictum is an agent of hyalohyphomycosis and has been identified as an increasingly frequent human pathogen in immunosuppressed individuals, causing localized, disseminated and invasive infections. Although extremely rare, A. strictum can infect immunocompetent individuals, as well as neonates. Due to the growing number of infections caused by A. strictum in the past few years, the need for new medical techniques in the identification of the fungus as well as for the treatment of human infections has risen considerably.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

Pseudallescheria boydii is a species of fungus classified in the Ascomycota. It is associated with some forms of eumycetoma/maduromycosis and is the causative agent of pseudallescheriasis. Typically found in stagnant and polluted water, it has been implicated in the infection of immunocompromised and near-drowned pneumonia patients. Treatment of infections with P. boydii is complicated by resistance to many of the standard antifungal agents normally used to treat infections by filamentous fungi.

Lomentospora prolificans is an emerging opportunistic fungal pathogen that causes a wide variety of infections in immunologically normal and immunosuppressed people and animals. It is resistant to most antifungal drugs and infections are often fatal. Drugs targeting the Class II dihydroorotate dehydrogenase (DHODH) proteins of L. prolificans, Scedosporium, Aspergillus and other deadly moulds are the basis for at least one new therapy, Olorofim, which is currently in phase 2b clinical trials and has received breakthrough status by FDA. For information on all DHODH proteins, please see Dihydroorotate dehydrogenase.
Phialemonium curvatum is a pathogenic fungus in the phylum Ascomycota. The genus was created to accommodate taxa intermediate to Acremonium and Phialophora. This genus is characterized by its abundance of adelophialides and few discrete phialides with no signs of collarettes. Specifically, P. curvatum is characterized by its grayish white colonies and its allantoid conidia. Phialemonium curvatum is typically found in a variety of environments including air, soil, industrial water and sewage. Furthermore, P. curvatum affects mainly immunocompromised and is rarely seen in immunocompetent people. The species has been known to cause peritonitis, endocarditis, endovascular infections, osteomyelitis as well as cutaneous infections of wounds and burns.
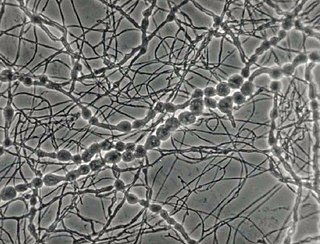
Madurella mycetomatis is a fungus primarily reported in Central Africa as a cause of mycetoma in humans. It has been misclassified for many years, but with improvement of molecular techniques, its phylogenetic classification has been established. Many methods exist to identify M. mycetomatis, both in lesions and in culture. Histological examination is especially useful, as it has many unique morphological features. Strain-level differences in response to antifungal agents is informative for treatment and laboratory isolation of cultures.

Phialemonium obovatum is a saprotrophic filamentous fungus able to cause opportunistic infections in humans with weakened immune systems. P. obovatum is widespread throughout the environment, occurring commonly in sewage, soil, air and water. Walter Gams and Michael McGinnis described the genus Phialemonium to accommodate species intermediate between the genera Acremonium and Phialophora. Currently, three species of Phialemonium are recognized of which P. obovatum is the only one to produce greenish colonies and obovate conidia. It has been investigated as one of several microfungi with potential use in the accelerated aging of wine.

Rhinocladiella mackenziei is a deeply pigmented mold that is a common cause of human cerebral phaeohyphomycosis. Rhinocladiella mackenziei was believed to be endemic solely to the Middle East, due to the first cases of infection being limited to the region. However, cases of R. mackenziei infection are increasingly reported from regions outside the Middle East. This pathogen is unique in that the majority of cases have been reported from immunologically normal people.
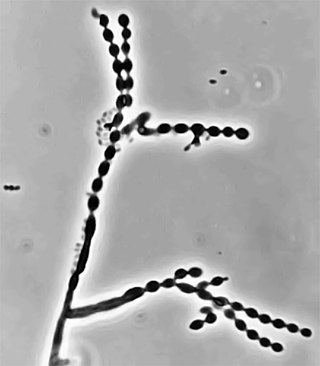
Cladophialophora carrionii is a melanized fungus in the genus Cladophialophora that is associated with decaying plant material like cacti and wood. It is one of the most frequent species of Cladophialophora implicated in human disease. Cladophialophora carrionii is a causative agent of chromoblastomycosis, a subcutaneous infection that occurs in sub-tropical areas such as Madagascar, Australia and northwestern Venezuela. Transmission occurs through traumatic implantation of plant material colonized by C. carrionii, mainly infecting rural workers. When C. carrionii infects its host, it transforms from a mycelial state to a muriform state to better tolerate the extreme conditions in the host's body.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
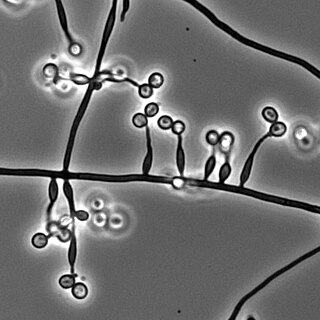
Metarhizium granulomatis is a fungus in the family Clavicipitaceae associated with systemic mycosis in veiled chameleons. The genus Metarhizium is known to infect arthropods, and collectively are referred to green-spored asexual pathogenic fungi. This species grows near the roots of plants and has been reported as an agent of disease in captive veiled chameleons. The etymology of the species epithet, "granulomatis" refers to the ability of the fungus to cause granulomatous disease in susceptible reptiles.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Arthrographis kalrae is an ascomycetous fungus responsible for human nail infections described in 1938 by Cochet as A. langeronii. A. kalrae is considered a weak pathogen of animals including human restricted to the outermost keratinized layers of tissue. Infections caused by this species are normally responsive to commonly used antifungal drugs with only very rare exceptions.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.












