A radioligand is a microscopic particle which consists of a therapeutic radioactive isotope and the cell-targeting compound - the ligand. The ligand is the target binding site, it may be on the surface of the targeted cancer cell for therapeutic purposes. Radioisotopes can occur naturally or be synthesized and produced in a cyclotron/nuclear reactor. The different types of radioisotopes include Y-90, H-3, C-11, Lu-177, Ac-225, Ra-223, In-111, I-131, I-125, etc. Thus, radioligands must be produced in special nuclear reactors for the radioisotope to remain stable. Radioligands can be used to analyze/characterize receptors, to perform binding assays, to help in diagnostic imaging, and to provide targeted cancer therapy. Radiation is a novel method of treating cancer and is effective in short distances along with being unique/personalizable and causing minimal harm to normal surrounding cells. Furthermore, radioligand binding can provide information about receptor-ligand interactions in vitro and in vivo. Choosing the right radioligand for the desired application is important. The radioligand must be radiochemically pure, stable, and demonstrate a high degree of selectivity, and high affinity for their target.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).
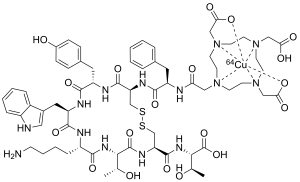
Copper-64 (64Cu) is a positron and beta emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography. Its unusually long half-life (12.7-hours) for a positron-emitting isotope makes it increasingly useful when attached to various ligands, for PET and PET-CT scanning.

An octreotide scan is a type of SPECT scintigraphy used to find carcinoid, pancreatic neuroendocrine tumors, and to localize sarcoidosis. It is also called somatostatin receptor scintigraphy (SRS). Octreotide, a drug similar to somatostatin, is radiolabeled with indium-111, and is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin. A gamma camera detects the radioactive octreotide, and makes pictures showing where the tumor cells are in the body, typically by a SPECT technique. A technetium-99m based radiopharmaceutical kit is also available.

DOTA-TATE is an eight amino acid long peptide, with a covalently bonded DOTA bifunctional chelator.
Caplacizumab is a bivalent single-domain antibody (VHH) designed for the treatment of thrombotic thrombocytopenic purpura (TTP) and thrombosis.

Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as atopic dermatitis (eczema), asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.
Advanced Accelerator Applications is a France-based pharmaceutical group, specialized in the field of nuclear medicine. The group operates in all three segments of nuclear medicine to diagnose and treat serious conditions in the fields of oncology, neurology, cardiology, infectious and inflammatory diseases.

Copanlisib, sold under the brand name Aliqopa, is a medication used for the treatment of adults experiencing relapsed follicular lymphoma who have received at least two prior systemic therapies.

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.
A PSMA scan is a nuclear medicine imaging technique used in the diagnosis and staging of prostate cancer. It is carried out by injection of a radiopharmaceutical with a positron or gamma emitting radionuclide and a prostate-specific membrane antigen (PSMA) targeting ligand. After injection, imaging of positron emitters such as gallium-68 (68Ga), copper-64 (64Cu), and fluorine-18 (18F) is carried out with a positron emission tomography (PET) scanner. For gamma emitters such as technetium-99m (99mTc) and indium-111 (111In) single-photon emission computed tomography (SPECT) imaging is performed with a gamma camera.

Zanubrutinib, sold under the brand name Brukinsa, is an anticancer medication used for the treatment of mantle cell lymphoma (MCL), Waldenström's macroglobulinemia (WM), marginal zone lymphoma (MZL), and chronic lymphocytic leukemia (CLL). Zanubrutinib is classified as a Bruton's tyrosine kinase (BTK) inhibitor. It is given by mouth.

Lutetium (177Lu) oxodotreotide (INN) or 177Lu dotatate, brand name Lutathera, is a chelated complex of a radioisotope of the element lutetium with dotatate, used in peptide receptor radionuclide therapy. Specifically, it is used in the treatment of cancers which express somatostatin receptors. It is a radiolabeled somatostatin analog.

Selpercatinib, sold under the brand name Retevmo among others, is a medication for the treatment of cancers in people whose tumors have an alteration in a specific gene. It is taken by mouth.

Flortaucipir (18F), sold under the brand name Tauvid, is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) imaging to image the brain.
Amivantamab, sold under the brand name Rybrevant, is a bispecific monoclonal antibody used to treat non-small cell lung cancer. Amivantamab is a bispecific epidermal growth factor (EGF) receptor-directed and mesenchymal–epithelial transition (MET) receptor-directed antibody. It is the first treatment for adults with non-small cell lung cancer whose tumors have specific types of genetic mutations: epidermal growth factor receptor (EGFR) exon 20 insertion mutations.

Piflufolastat F-18, sold under the brand name Pylarify among others, is a radioactive diagnostic agent used for positron emission tomography (PET) imaging. It is given by intravenous injection.

Gallium (68Ga) gozetotide or Gallium (68Ga) PSMA-11 sold under the brand name Illuccix among others, is a radiopharmaceutical made of 68Ga conjugated to prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx)-HBED-CC, used for imaging prostate cancer by positron emission tomography (PET). The PSMA targeting ligand specifically directs the radiolabeled imaging agent towards the prostate cancerous lesions in men.