
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements.

Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.
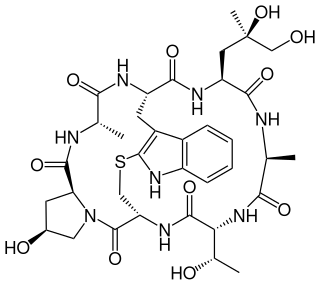
Phalloidin belongs to a class of toxins called phallotoxins, which are found in the death cap mushroom (Amanita phalloides). It is a rigid bicyclic heptapeptide that is lethal after a few days when injected into the bloodstream. The major symptom of phalloidin poisoning is acute hunger due to the destruction of liver cells. It functions by binding and stabilizing filamentous actin (F-actin) and effectively prevents the depolymerization of actin fibers. Due to its tight and selective binding to F-actin, derivatives of phalloidin containing fluorescent tags are used widely in microscopy to visualize F-actin in biomedical research.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

Cytochalasin B, the name of which comes from the Greek cytos (cell) and chalasis (relaxation), is a cell-permeable mycotoxin. It was found that substoichimetric concentrations of cytochalasin B (CB) strongly inhibit network formation by actin filaments. Due to this, it is often used in cytological research. It inhibits cytoplasmic division by blocking the formation of contractile microfilaments. It inhibits cell movement and induces nuclear extrusion. Cytochalasin B shortens actin filaments by blocking monomer addition at the fast-growing end of polymers. Cytochalasin B inhibits glucose transport and platelet aggregation. It blocks adenosine-induced apoptotic body formation without affecting activation of endogenous ADP-ribosylation in leukemia HL-60 cells. It is also used in cloning through nuclear transfer. Here enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Thereby, the oocyte genome is removed from the oocyte, while preventing rupture of the plasma membrane.
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton. MAPs are integral to: the stability of the cell and its internal structures and the transport of components within the cell
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemical signals and mechanical signals. Errors during this process have serious consequences, including intellectual disability, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells.

A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements". Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure.
ADF/cofilin is a family of actin-binding proteins associated with the rapid depolymerization of actin microfilaments that give actin its characteristic dynamic instability. This dynamic instability is central to actin's role in muscle contraction, cell motility and transcription regulation.
Cytochalasins are fungal metabolites that have the ability to bind to actin filaments and block polymerization and the elongation of actin. As a result of the inhibition of actin polymerization, cytochalasins can change cellular morphology, inhibit cellular processes such as cell division, and even cause cells to undergo apoptosis. Cytochalasins have the ability to permeate cell membranes, prevent cellular translocation and cause cells to enucleate. Cytochalasins can also have an effect on other aspects of biological processes unrelated to actin polymerization. For example, cytochalasin A and cytochalasin B can also inhibit the transport of monosaccharides across the cell membrane, cytochalasin H has been found to regulate plant growth, cytochalasin D inhibits protein synthesis and cytochalasin E prevents angiogenesis.
The latrunculins are a family of natural products and toxins produced by certain sponges, including genus Latrunculia and Negombata, whence the name is derived. It binds actin monomers near the nucleotide binding cleft with 1:1 stoichiometry and prevents them from polymerizing. Administered in vivo, this effect results in disruption of the actin filaments of the cytoskeleton, and allows visualization of the corresponding changes made to the cellular processes. This property is similar to that of cytochalasin, but has a narrow effective concentration range. Latrunculin has been used to great effect in the discovery of cadherin distribution regulation and has potential medical applications. Latrunculin A, a type of the toxin, was found to be able to make reversible morphological changes to mammalian cells by disrupting the actin network.
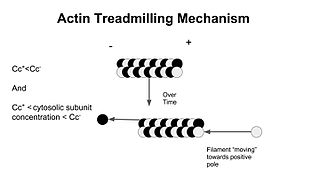
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling.
CapZ, also known as CAPZ, CAZ1 and CAPPA1, is a capping protein that caps the barbed end of actin filaments in muscle cells.
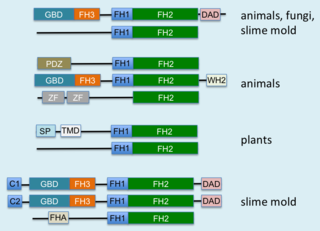
Formins are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus.
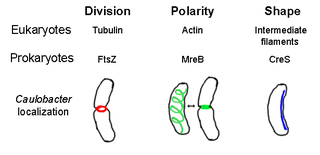
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but advances in visualization technology and structure determination led to the discovery of filaments in these cells in the early 1990s. Not only have analogues for all major cytoskeletal proteins in eukaryotes been found in prokaryotes, cytoskeletal proteins with no known eukaryotic homologues have also been discovered. Cytoskeletal elements play essential roles in cell division, protection, shape determination, and polarity determination in various prokaryotes.
Catastrophin is a term use to describe proteins that are associated with the disassembly of microtubules. Catastrophins affect microtubule shortening, a process known as microtubule catastrophe.
ParM is a prokaryotic actin homologue which provides the force to drive copies of the R1 plasmid to opposite ends of rod shaped bacteria before cytokinesis.
Actin remodeling is the biochemical process that allows for the dynamic alterations of cellular organization. The remodeling of actin filaments occurs in a cyclic pattern on cell surfaces and exists as a fundamental aspect to cellular life. During the remodeling process, actin monomers polymerize in response to signaling cascades that stem from environmental cues. The cell's signaling pathways cause actin to affect intracellular organization of the cytoskeleton and often consequently, the cell membrane. Again triggered by environmental conditions, actin filaments break back down into monomers and the cycle is completed. Actin-binding proteins (ABPs) aid in the transformation of actin filaments throughout the actin remodeling process. These proteins account for the diverse structure and changes in shape of Eukaryotic cells. Despite its complexity, actin remodeling may result in complete cytoskeletal reorganization in under a minute.












