
Centrarchidae, better known as sunfishes, is a family of freshwater ray-finned fish belonging to the order Perciformes, native only to North America. There are eight universally included genera within the centrarchid family: Lepomis, Micropterus, Pomoxis (crappies), Enneacanthus, Centrarchus, Archoplites, Ambloplites, and Acantharchus. A genetic study in 2012 suggests that the highly distinct pygmy sunfishes of the genus Elassoma are also centrarchids.
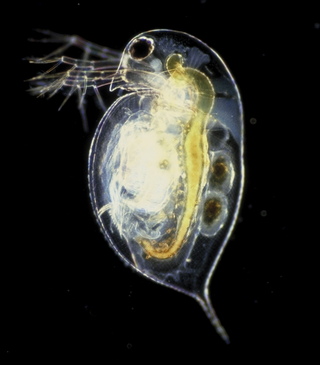
Daphnia is a genus of small planktonic crustaceans, 0.2–6.0 mm (0.01–0.24 in) in length. Daphnia are members of the order Anomopoda, and are one of the several small aquatic crustaceans commonly called water fleas because their saltatory swimming style resembles the movements of fleas. Daphnia spp. live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.

The bigmouth buffalo is a fish native to North America. It is the largest North American species in the Catostomidae or "sucker" family, and is one of the longest-lived and latest-maturing freshwater fishes, capable of living 127 years and reproducing infrequently. Even at a century old they show no age-related declines, but instead improvements relative to younger individuals, making this species a biological marvel. It is commonly called the gourdhead, marblehead, redmouth buffalo, buffalofish, bernard buffalo, roundhead, or brown buffalo. The bigmouth buffalo is not a carp, nor is any other fish in the sucker family. Although they share the same order, each belong to different suborders and are native to separate continents.

Freshwater fish are those that spend some or all of their lives in fresh water, such as rivers and lakes, with a salinity of less than 1.05%. These environments differ from marine conditions in many ways, especially the difference in levels of salinity. To survive fresh water, the fish need a range of physiological adaptations.

Cercopagis pengoi, or the fishhook waterflea, is a species of planktonic cladoceran crustaceans that is native in the brackish fringes of the Black Sea and the Caspian Sea. In recent decades it has spread as an invasive species to some freshwater waterways and reservoirs of Eastern Europe and to the brackish Baltic Sea. Further it was introduced in ballast water to the Great Lakes of North America and a number of adjacent lakes, and has become a pest classified among the 100 worst invasive species of the world.

Gammarus roeselii is a species of freshwater amphipod native to Europe.

Bythotrephes longimanus, or the spiny water flea, is a planktonic crustacean less than 15 millimetres (0.6 in) long. It is native to fresh waters of Northern Europe and Asia, but has been accidentally introduced and widely distributed in the Great Lakes area of North America since the 1980s. Bythotrephes is typified by a long abdominal spine with several barbs which protect it from predators.

The white-faced darter or small whiteface is a dragonfly belonging to the genus Leucorrhinia in the family Libellulidae, characterised by red and black markings and a distinctive white patch on the head. It is found in wetlands and peat bogs from northern Europe eastwards to Siberia, and the adults are active from around April till September, which is known as the "flight period". It breeds in acidic bodies of water, laying its eggs in clumps of sphagnum moss that provide a safe habitat for larval development. The larvae are particularly vulnerable to predation by fish, and so are usually found in lakes where fish are not present. L. dubia is listed as a species of least concern (LC) by the IUCN Red List, however, it is potentially threatened by habitat destruction, pollution, and climate change.

The Diplostraca or Cladocera, commonly known as water fleas, is a superorder of small, mostly freshwater crustaceans, most of which feed on microscopic chunks of organic matter, though some forms are predatory.

Daphnia pulex is the most common species of water flea. It has a cosmopolitan distribution: the species is found throughout the Americas, Europe, and Australia. It is a model species, and was the first crustacean to have its genome sequenced.
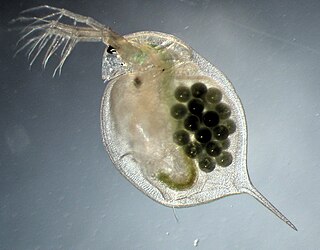
Daphnia magna is a small planktonic crustacean that belongs to the subclass Phyllopoda.
Ceriodaphnia dubia is a species of water flea in the class Branchiopoda, living in freshwater lakes, ponds, and marshes in most of the world. They are small, generally less than 1 millimetre (0.039 in) in length. Males are smaller than females. C. dubia moves using a powerful set of second antennae, and is used in toxicity testing of wastewater treatment plant effluent water in the United States. Climate change and particularly ultraviolet radiation B may seriously damage C. dubia populations, as they seems to be more sensitive than other cladocerans such as Daphnia pulex or D. pulicaria.

Leptodora is a genus containing two species of large, nearly transparent predatory water fleas. They grow up to 21 mm (0.83 in) long, with two large antennae used for swimming and a single compound eye. The legs are used to catch copepods that it comes into contact with by chance. Leptodora kindtii is found in temperate lakes across the Northern Hemisphere and is probably the only water flea species ever described in a newspaper; L. richardi is only known from eastern Russia. For most of the year, Leptodora reproduces parthenogenetically, with males only appearing late in the season, to produce winter eggs which hatch the following spring. Leptodora is the only genus in its family, the Leptodoridae, and suborder, Haplopoda.

Dikerogammarus villosus, also known as the killer shrimp, is a species of amphipod crustacean native to the Ponto-Caspian region of eastern Europe, but which has become invasive across the western part of the continent. In the areas it has invaded, it lives in a wide range of habitats and will prey on many other animals. It is fast-growing, reaching sexual maturity in 4–8 weeks. As it has moved through Europe, it threatens other species and has already displaced both native amphipods and previous invaders.

Notonecta maculata is a backswimmer of the family Notonectidae, found in Europe, including the United Kingdom.
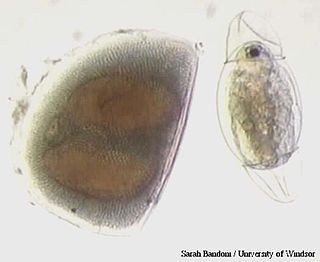
Ephippia are winter or dry-season eggs of the various species of small crustacean in the order Cladocera ; they are provided with an extra shell layer, which preserves and protects the resting stages inside from harsh environmental conditions until the more favorable times, such as spring, when the reproductive cycle is able to take place once again. Ephippia are part of the back of a mother carrying them until they are fully developed. After molting, the ephippium stays in the water, or in the soil of dried puddles, small ponds, and vernal pools. The resting stages are often called eggs, but are in fact embryos with arrested development. Ephippia can rest for many years before the embryo resumes development upon an appropriate hatching stimulus.
Carla Cáceres is a professor at the University of Illinois Urbana-Champaign known for her research in population, community and evolutionary ecology, focusing on the origins, maintenance, and functional significance of biodiversity within ecosystems. She is a Fellow of the American Association for the Advancement of Science, the Ecological Society of America, and the Association for the Sciences of Limnology and Oceanography

Ambassis macleayi, commonly known as Macleay's glassfish, Macleay's glass perchlet, Macleay's perchlet, reticulated glassfish, reticulated perchlet, or network perchlet, is a species of freshwater fish in the family Ambassidae. It is native to northern Australia and the trans-Fly River region of New Guinea. It is a fish with a vertically flat, narrow body and a standard length generally between 35 and 45 mm, with large specimens reaching 77 mm (3.0 in) long. It generally eats water fleas and other small invertebrates. This fish is considered to be a least-concern species according to the International Union for Conservation of Nature (IUCN), although it could suffer from habitat degradation due to feral pigs and invasive water plants such as the water hyacinth. The fish is suitable for aquarium use in tanks containing other non-aggressive species.

Daphnia longispina is a planktonic crustacean of the family Daphniidae, a cladoceran freshwater water flea. It is native to Eurasia. D. longispina is similar in size and sometimes confused with the often sympatric D. pulex, but much smaller than D. magna. D. longispina is found in a wide range of standing freshwater bodies from small, ephemeral rock-pools to large lakes.
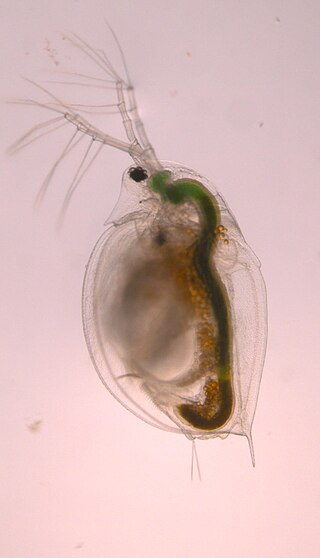
Daphnia pulicaria is a species of freshwater crustaceans found within the genus of Daphnia, which are often called "water fleas," and they are commonly used as model organisms for scientific research Like other species of Daphnia, they reproduce via cyclic parthenogenesis. D. pulicaria are filter-feeders with a diet primarily consisting of algae, including Ankistrodesmus falcatus, and they can be found in deep lakes located in temperate climates. Furthermore, D. pulicaria are ecologically important herbivorous zooplankton, which help control algal populations and are a source of food for some fish. D. pulicaria are closely related to Daphnia pulex, and numerous studies have investigated the nature and strength of this relationship because these species can produce Daphnia pulex-pulicaria hybrids. In recent years, D. pulicaria along with other Daphnia species have been negatively affected by invasive predators, such as Bythotrephes longimanus.















