![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)
In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane. They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, grease, rubber, resin, and caulk.

Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water.
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution.
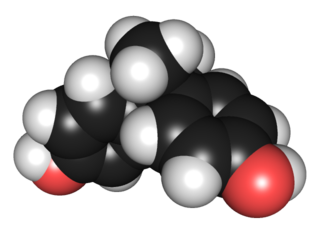
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.

Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.
Silicone grease, sometimes called dielectric grease, is a waterproof grease made by combining a silicone oil with a thickener. Most commonly, the silicone oil is polydimethylsiloxane (PDMS) and the thickener is amorphous fumed silica. Using this formulation, silicone grease is a translucent white viscous paste, with exact properties dependent on the type and proportion of the components. More specialized silicone greases are made from fluorinated silicones or, for low-temperature applications, PDMS containing some phenyl substituents in place of methyl groups. Other thickeners may be used, including stearates and powdered polytetrafluorethylene (PTFE). Greases formulated from silicone oils with silica thickener are sometimes referred to as silicone paste to distinguish them from silicone grease made with silicone oil and a soap thickener.

The European Chemicals Agency is an agency of the European Union working for the safe use of chemicals. It manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ECHA is the driving force among regulatory authorities in implementing the EU's chemicals legislation. ECHA has to ascertain that companies comply with the legislation, advances the safe use of chemicals, provides information on chemicals and addresses chemicals of concern. It is located in Helsinki, Finland. ECHA is an independent and mature regulatory agency established by REACH. It is not a subsidiary entity of the European Commission.

Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Hexamethyldisiloxane (HMDSO or MM) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
A silicone oil is any liquid polymerized siloxane with organic side chains. The most important member is polydimethylsiloxane. These polymers are of commercial interest because of their relatively high thermal stability and their lubricating properties.
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.
Dimethyldichlorosilane is a tetrahedral organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds.

Octamethylcyclotetrasiloxane, also called D4, is an organosilicon compound with the formula [(CH3)2SiO]4. It is a colorless viscous liquid. It is a common cyclomethicone. It is widely used in cosmetics.
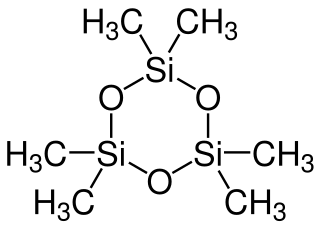
Hexamethylcyclotrisiloxane, also known as D3 and D3, is the organosilicon compound with the formula [(CH3)2SiO]3. It is a colorless or white volatile solid. It finds limited use in organic chemistry. The larger tetrameric and pentameric siloxanes, respectively octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane, are of significant industrial interest, whereas 1,000–10,000 tonnes per year of the trimer is manufactured and/or imported in the European Economic Area.

Hexa(methoxymethyl)melamine (HMMM) is a hemiaminal ether commonly used as a crosslinking agent in the production of coatings and rubber items. It is produced via the reaction of melamine with formaldehyde and excess methanol, with the later also acting as a solvent for the reaction. It can be considered as a monomeric intermediate in the formation of melamine resin.





![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)









