
Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.

Dinitrophenols are chemical compounds which are nitro derivatives of phenol.
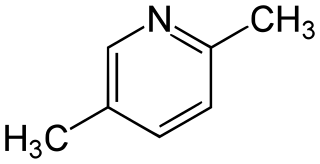
Lutidine is the trivial name used to describe the chemical compounds which are dimethyl derivatives of pyridine. They were discovered in Dippel's oil and named by Thomas Anderson in 1851. Their chemical properties resemble those of pyridine, although the presence of the methyl groups may prohibit some of the more straightforward reactions. Lutidine comes in several isomers:
A chlorophenol is any organochloride of phenol that contains one or more covalently bonded chlorine atoms. There are five basic types of chlorophenols and 19 different chlorophenols in total when positional isomerism is taken into account. Chlorophenols are produced by electrophilic halogenation of phenol with chlorine.
In enzymology, a 2,4-dichlorophenol 6-monooxygenase (EC 1.14.13.20) is an enzyme that catalyzes the chemical reaction
A trichlorophenol is any organochloride of phenol that contains three covalently bonded chlorine atoms. Trichlorophenols are produced by electrophilic halogenation of phenol with chlorine. Different isomers of trichlorophenol exist according to which ring positions on the phenol contain chlorine atoms. 2,4,6-Trichlorophenol, for example, has two chlorine atoms in the ortho positions and one chlorine atom in the para position.
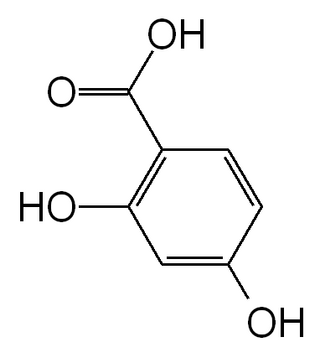
Dihydroxybenzoic acids (DHBA) are a type of phenolic acids.
2,4-Dichlorophenol (2,4-DCP) is a chlorinated derivative of phenol with the molecular formula Cl2C6H3OH. It is a white solid that is mildly acidic (pKa = 7.9). It is produced on a large scale as a precursor to the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D).
2,6-Dichlorophenol is a compound with formula C6H3Cl2OH. It is one of the six isomers of dichlorophenol. It is a colorless solid. Its pKa is 6.78, which is about 100x more acidic than 2-chlorophenol (8.52) and 1000x more acidic than phenol itself (9.95).
The molecular formula C6H4Cl2O could refer to:
Dinitrotoluenes could refer to one of the following compounds:

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.
Dihydroxycinnamic acid may refer to several molecules with the molecular formula C9H8O4 including:
Aquamicrobium lusatiense is a Gram-negative, oxidase-positive, strictly aerobic bacteria from the genus Aquamicrobium with a polar flagellum, which was isolated from activated sludge in Germany. Aquamicrobium lusatiense is able to degrade 2,4-dichlorophenol, 4-chloro-2-methylphenol, 4-chlorophenol, and phenol.

Toluenediamine may refer to these isomeric organic compounds with the formula C6H3(NH2)2(CH3):

3-Chlorophenol is an organic compound with the molecular formula C6H4ClOH. It is one of three isomers of monochlorophenol. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Together with 3,5-dichlorophenol, it is prepared industrially by dechlorination of polychlorophenols. Alternatively, it arises via the cumene process, which starts with the alkylation of chlorobenzene with propylene.

Ohio River Park is a Superfund Site located in Neville Island, Pennsylvania. Between the 1920s-1970s, the Site was used for municipal waste, pesticide manufacturing, coke sludge disposal, cement manufacturing disposal, and pesticide waste. In 1977, Neville Land Company donated the Site to Allegheny County who started developing the Site as a community park. In 1979, Allegheny County found various hazardous contaminants on the Site. On August 30, 1990, the Site was determined to be a Superfund Site due to VOCs, SVOCs, inorganics, and pesticides being present in the surface soil, subsurface soil, surface water, river sediment, and groundwater. Soil remediation began in February 1998 and ended in September 1999. Today, Ohio River Park has the Robert Morris University Island Sports Center and Coraopolis Bridge on top of it. Additionally, benzene continues to be monitored because it is still present in the Site's groundwater.
A tetrachlorophenol is any organochloride of phenol that contains four covalently bonded chlorine atoms. Tetrachlorophenols are produced by electrophilic halogenation of phenol with chlorine. Different isomers of tetrachlorophenol exist according to which ring positions on the phenol contain chlorine atoms.








