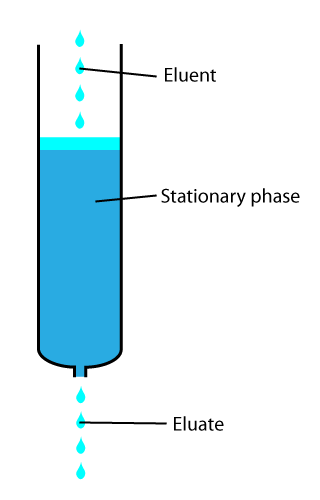
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Column chromatography in chemistry is a chromatography method used to isolate a single chemical compound from a mixture. Chromatography is able to separate substances based on differential adsorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.

Fast protein liquid chromatography (FPLC) is a form of liquid chromatography that is often used to analyze or purify mixtures of proteins. As in other forms of chromatography, separation is possible because the different components of a mixture have different affinities for two materials, a moving fluid and a porous solid. In FPLC the mobile phase is an aqueous buffer solution. The buffer flow rate is controlled by a positive-displacement pump and is normally kept constant, while the composition of the buffer can be varied by drawing fluids in different proportions from two or more external reservoirs. The stationary phase is a resin composed of beads, usually of cross-linked agarose, packed into a cylindrical glass or plastic column. FPLC resins are available in a wide range of bead sizes and surface ligands depending on the application.
Reversed-phase liquid chromatography (RP-LC) is a mode of liquid chromatography in which non-polar stationary phase and polar mobile phases are used for the separation of organic compounds. The vast majority of separations and analyses using high-performance liquid chromatography (HPLC) in recent years are done using the reversed phase mode. In the reversed phase mode, the sample components are retained in the system the more hydrophobic they are.
Mixed-mode chromatography (MMC), or multimodal chromatography, refers to chromatographic methods that utilize more than one form of interaction between the stationary phase and analytes in order to achieve their separation. What is distinct from conventional single-mode chromatography is that the secondary interactions in MMC cannot be too weak, and thus they also contribute to the retention of the solutes.
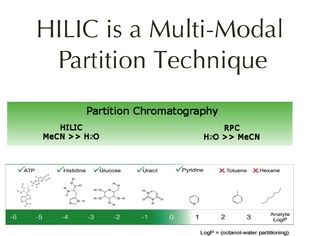
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Chromatography is a physical method of separation that distributes the components you want to separate between two phases, one stationary, the other moving in a definite direction. Cold ethanol precipitation, developed by Cohn in 1946, manipulates pH, ionic strength, ethanol concentration and temperature to precipitate different protein fractions from plasma. Chromatographic techniques utilise ion exchange, gel filtration and affinity resins to separate proteins. Since the 1980s it has emerged as an effective method of purifying blood components for therapeutic use.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.
Aqueous normal-phase chromatography (ANP) is a chromatographic technique that involves the mobile phase compositions and polarities between reversed-phase chromatography (RP) and normal-phase chromatography (NP), while the stationary phases are polar.
Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) is a form of chromatography that is used to separate or purify biomolecules from complex mixtures. It was developed at the Swiss Federal Institute of Technology Zürich by Aumann and Morbidelli. The process consists of two to six chromatographic columns which are connected to one another in such a way that as the mixture moves through the columns the compound is purified into several fractions.
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Solvent impregnated resins (SIRs) are commercially available (macro)porous resins impregnated with a solvent/an extractant. In this approach, a liquid extractant is contained within the pores of (adsorption) particles. Usually, the extractant is an organic liquid. Its purpose is to extract one or more dissolved components from a surrounding aqueous environment. The basic principle combines adsorption, chromatography and liquid-liquid extraction.
Thermoresponsive polymers can be used as stationary phase in liquid chromatography. Here, the polarity of the stationary phase can be varied by temperature changes, altering the power of separation without changing the column or solvent composition. Thermally related benefits of gas chromatography can now be applied to classes of compounds that are restricted to liquid chromatography due to their thermolability. In place of solvent gradient elution, thermoresponsive polymers allow the use of temperature gradients under purely aqueous isocratic conditions. The versatility of the system is controlled not only through changing temperature, but through the addition of modifying moieties that allow for a choice of enhanced hydrophobic interaction, or by introducing the prospect of electrostatic interaction. These developments have already introduced major improvements to the fields of hydrophobic interaction chromatography, size exclusion chromatography, ion exchange chromatography, and affinity chromatography separations as well as pseudo-solid phase extractions.









