Related Research Articles

Long QT syndrome (LQTS) is a condition affecting repolarization (relaxing) of the heart after a heartbeat, giving rise to an abnormally lengthy QT interval. It results in an increased risk of an irregular heartbeat which can result in fainting, drowning, seizures, or sudden death. These episodes can be triggered by exercise or stress. Some rare forms of LQTS are associated with other symptoms and signs including deafness and periods of muscle weakness.
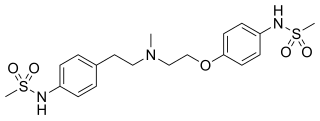
Dofetilide is a class III antiarrhythmic agent. It is marketed under the trade name Tikosyn by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 µg of dofetilide. It is not available in Europe or Australia.
Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.
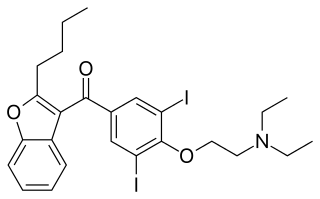
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias. This includes ventricular tachycardia (VT), ventricular fibrillation (VF), and wide complex tachycardia, as well as atrial fibrillation and paroxysmal supraventricular tachycardia. Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin.
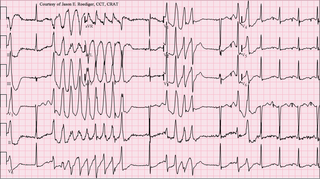
Torsades de pointes, torsade de pointes or torsades des pointes is a specific type of abnormal heart rhythm that can lead to sudden cardiac death. It is a polymorphic ventricular tachycardia that exhibits distinct characteristics on the electrocardiogram (ECG). It was described by French physician François Dessertenne in 1966. Prolongation of the QT interval can increase a person's risk of developing this abnormal heart rhythm, occurring in between 1% and 10% of patients who receive QT-prolonging antiarrhythmic drugs.
Ventricular tachycardia is a cardiovascular disorder in which fast heart rate occurs in the ventricles of the heart. Although a few seconds of VT may not result in permanent problems, longer periods are dangerous; and multiple episodes over a short period of time are referred to as an electrical storm. Short periods may occur without symptoms, or present with lightheadedness, palpitations, shortness of breath, chest pain, and decreased level of consciousness. Ventricular tachycardia may lead to coma and persistent vegetative state due to lack of blood and oxygen to the brain. Ventricular tachycardia may result in ventricular fibrillation (VF) and turn into cardiac arrest. This conversion of the VT into VF is called the degeneration of the VT. It is found initially in about 7% of people in cardiac arrest.
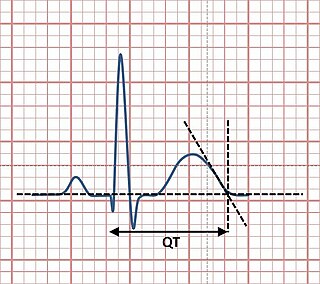
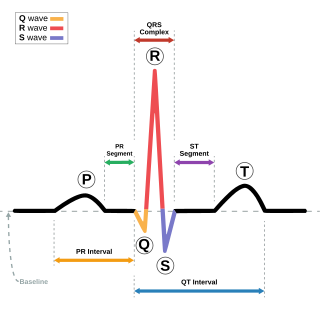
The QT interval is a measurement made on an electrocardiogram used to assess some of the electrical properties of the heart. It is calculated as the time from the start of the Q wave to the end of the T wave, and approximates to the time taken from when the cardiac ventricles start to contract to when they finish relaxing. An abnormally long or abnormally short QT interval is associated with an increased risk of developing abnormal heart rhythms and sudden cardiac death. Abnormalities in the QT interval can be caused by genetic conditions such as long QT syndrome, by certain medications such as sotalol or pitolisant, by disturbances in the concentrations of certain salts within the blood such as hypokalaemia, or by hormonal imbalances such as hypothyroidism.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.
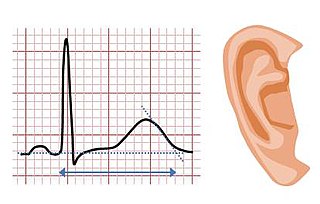
Jervell and Lange-Nielsen syndrome (JLNS) is a rare type of long QT syndrome associated with severe, bilateral sensorineural hearing loss. Those with JLNS are at risk of abnormal heart rhythms called arrhythmias, which can lead to fainting, seizures, or sudden death. JLNS, like other forms of long QT syndrome, causes the cardiac muscle to take longer than usual to recharge between beats. It is caused by genetic variants responsible for producing ion channels that carry transport potassium out of cells. The condition is usually diagnosed using an electrocardiogram, but genetic testing can also be used. Treatment includes lifestyle measures, beta blockers, and implantation of a defibrillator in some cases. It was first described by Anton Jervell and Fred Lange-Nielsen in 1957.

Romano–Ward syndrome is the most common form of congenital Long QT syndrome (LQTS), a genetic heart condition that affects the electrical properties of heart muscle cells. Those affected are at risk of abnormal heart rhythms which can lead to fainting, seizures, or sudden death. Romano–Ward syndrome can be distinguished clinically from other forms of inherited LQTS as it affects only the electrical properties of the heart, while other forms of LQTS can also affect other parts of the body.

Andersen–Tawil syndrome, also called Andersen syndrome and long QT syndrome 7, is a rare genetic disorder affecting several parts of the body. The three predominant features of Andersen–Tawil syndrome include disturbances of the electrical function of the heart characterised by an abnormality seen on an electrocardiogram and a tendency to abnormal heart rhythms, physical characteristics including low-set ears and a small lower jaw, and intermittent periods of muscle weakness known as hypokalaemic periodic paralysis.

hERG is a gene that codes for a protein known as Kv11.1, the alpha subunit of a potassium ion channel. This ion channel is best known for its contribution to the electrical activity of the heart: the hERG channel mediates the repolarizing IKr current in the cardiac action potential, which helps coordinate the heart's beating.

Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis. It was approved by the US Food and Drug Administration (FDA) in July 2009. Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. The FDA label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in people with moderate to severe congestive heart failure.

Ranolazine, sold under the brand name Ranexa among others, is a medication used to treat heart related chest pain. Typically it is used together with other medications when those are insufficient. Therapeutic benefits appear smaller in females than males. It is taken by mouth.

Potassium voltage-gated channel subfamily E member 2 (KCNE2), also known as MinK-related peptide 1 (MiRP1), is a protein that in humans is encoded by the KCNE2 gene on chromosome 21. MiRP1 is a voltage-gated potassium channel accessory subunit associated with Long QT syndrome. It is ubiquitously expressed in many tissues and cell types. Because of this and its ability to regulate multiple different ion channels, KCNE2 exerts considerable influence on a number of cell types and tissues. Human KCNE2 is a member of the five-strong family of human KCNE genes. KCNE proteins contain a single membrane-spanning region, extracellular N-terminal and intracellular C-terminal. KCNE proteins have been widely studied for their roles in the heart and in genetic predisposition to inherited cardiac arrhythmias. The KCNE2 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease. More recently, roles for KCNE proteins in a variety of non-cardiac tissues have also been explored.
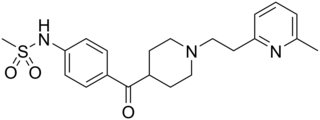
E-4031 is an experimental class III antiarrhythmic drug that blocks potassium channels of the hERG-type.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.

AZD1305 is an experimental drug candidate that is under investigation for the management and reversal of cardiac arrhythmias, specifically atrial fibrillation and flutter. In vitro studies have shown that this combined-ion channel blocker inhibits rapidly the activating delayed-rectifier potassium current (IKr), L-type calcium current, and inward sodium current (INa).
CredibleMeds is an online database launched in 2009 of information regarding serious drug-drug interactions associated with QT prolongation or the potentially lethal arrhythmia, torsades de pointes (TdP). It also assists with measurement of the quality of healthcare delivery for the Centers for Medicare and Medicaid Services, and aids in the management of patients with inherited channelopathies.
References
- ↑ Roden DM, Woosley RL, Primm RK (1986). "Incidence and clinical features of the quinidine-associated long QT syndrome: Implications for care". American Heart Journal. 111 (6): 1088–93. doi:10.1016/0002-8703(86)90010-4. PMID 3716982.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 3 Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE (2012). "Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review". Scientific World Journal. 2012: 212178. doi: 10.1100/2012/212178 . PMC 3347892 . PMID 22593664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 3 4 Olsen, K. M. (2005). "Pharmacologic agents associated with QT interval prolongation". The Journal of Family Practice. Suppl (Suppl): S8–S14. PMID 15938993.
- ↑ Hohnloser, SH; Singh, BN (1995). "Proarrhythmia with class III antiarrhythmic drugs: definition, electrophysiologic mechanisms, incidence, predisposing factors, and clinical implications". Cardiovascular Electrophysiology. 6 (10 Pt 2): 920–36. doi:10.1111/j.1540-8167.1995.tb00368.x. PMID 8548113. S2CID 23136846.
- ↑ Ben-David, J; Zipes, DP (1993). "Torsades de pointes and proarrhythmia". Lancet. 341 (8860): 1578–1582. doi:10.1016/0140-6736(93)90708-o. PMID 8099651. S2CID 44309083.
- 1 2 Khan IA (2002). "Long QT syndrome: diagnosis and management". American Heart Journal. 143 (1): 7–14. doi:10.1067/mhj.2002.120295. PMID 11773906.
- 1 2 3 Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE (2012). "Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review". Scientific World Journal. 2012: 212178. doi: 10.1100/2012/212178 . PMC 3347892 . PMID 22593664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Tzivoni, D; Schuger, C; Benhorin, J; Keren, A; Stern, S (1988). "Treatment of torsade de pointes with magnesium sulfate". Circulation. 77 (2): 392–397. doi: 10.1161/01.cir.77.2.392 . PMID 3338130.
- ↑ El-Sherif N, Turitto G, Boutjdir M (2018). "Acquired long QT syndrome and torsade de pointes". Pacing and Clinical Electrophysiology. 41 (4): 414–421. doi:10.1111/pace.13296. PMID 29405316. S2CID 46795997.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Molokhia M, Pathak A, Lapeyre-Mestre M; et al. (2008). "Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France". British Journal of Clinical Pharmacology. 66 (3): 386–95. doi:10.1111/j.1365-2125.2008.03229.x. PMC 2526236 . PMID 18637888.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Straus, SM; Sturkenboom, MC; Bleumink, GS; Dieleman, JP; van der Lei, J; de Graeff, PA; Kingma, JH; Stricker, BH (2005). "Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death". European Heart Journal. 26 (19): 2007–12. doi: 10.1093/eurheartj/ehi312 . PMID 15888497.