Attention
Neural substrates
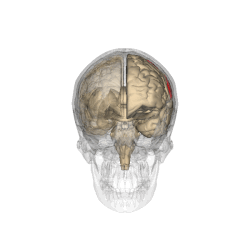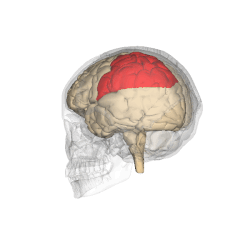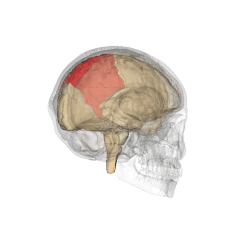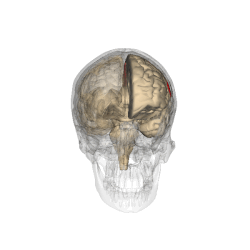
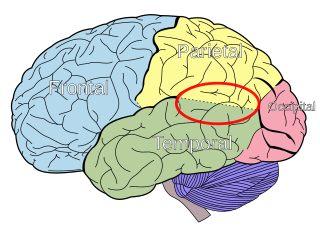
The parietal lobes of the brain are largely involved in attention. Lesions to this region of the brain in humans result in difficulty or inability to attend to events that are contralateral to the lesioned hemisphere. Those with lesions to the posterior parietal lobe have little to no difficulty shifting attention to and from stimuli appearing in the space ipsilateral to the lesioned hemisphere. However, they do display a slowed response in shifting their focus of current attention to events and stimuli appearing contralateral to the lesioned hemisphere. [4]
Studies involving single-unit recordings from the parietal lobes of monkeys have indicated that there are neurons solely involved in integrating visual spatial information with postural information. [5] Without this apparent combining of spatial information, it would be difficult or impossible to locate objects in external space (as information provided solely by the retina is insufficient). The position of the eyes, head and body must also be taken into consideration.
In addition, studies involving transcranial magnetic stimulation application over the parietal lobes as well as positron emission tomography (PET) analysis of the parietal lobes suggest that this region is involved in conjunction searches, but not in single-feature searches (see Visual search for supplementary information). [6]
Auditory attention

Auditory attention has been examined following sleep deprivation. In one study, researchers examined the auditory attention of twelve non-sleep-deprived subjects and twelve sleep-deprived subjects at various time intervals. Subjects were involved in an auditory attention task, which required the reproduction of the spatial relationships between four letters, using a graph composed of six squares, immediately following the presentation of an item from a tape recorder. It was found that auditory attention of sleep-deprived individuals is affected as the total amount of sleep-deprivation increases, possibly due to lowered perceptual vigilance. [7]
Divided attention
Functional magnetic resonance imaging (fMRI) scans of the brains of subjects exposed to thirty-five hours of sleep deprivation indicate that sleep deprivation is related to increases in prefrontal cortex and parietal lobe activation during tasks that combine verbal learning and arithmetic. This is particularly apparent in the right hemisphere. In non-sleep-deprived people involved in verbal learning and arithmetic tasks, the anterior cingulate cortex and the right prefrontal cortex are active. Following sleep deprivation, there is increased activation of the left inferior frontal gyrus and the bilateral parietal lobes. This information suggests that divided attention tasks require more attentional resources than normally required by a non-sleep-deprived person. [8]
Exogenous and endogenous attention
Studies using event-related potential (ERP) recordings have found that twenty-four hours of sleep deprivation decreases ERP response to signal inputs from endogenous, but not exogenous, sources. Therefore, it is suggested that sleep deprivation affects endogenously-driven selective attention to a greater extent than exogenously-driven selected attention. [9]
Selective attention
Twenty-four hours of sleep deprivation has been found to affect the functional connectivity between the inferior frontal parietal region (IPS) and the parahippocampal place area (PPA). However, sleep deprivation does not affect the attention modulation index of the PPA. With this information, researchers have concluded that the psychophysiological interaction (PPI) is more involved in selective attention than the IPS and PPA. [10]
Research has found that together, attention and sleep deprivation modulate the parahippocampal place area (PPA) activation and scene processing. Specifically, sleep deprivation was related to significant decreases in PPA activation while attending to scenes and when ignoring scenes. This is explained by the absence of change in the Attention Modulation Index (AMI). Face recognition is not affected by sleep deprivation. [10]
Sleep deprivation has been shown to negatively affect picture classification speed and accuracy, as well as recognition memory. [10] It results in an inability to avoid attending to irrelevant information displayed during attention-related tasks. (Norton) It also decreases activation in the ventral visual area and the frontal parietal control regions. [10]
Supervisory attention
Studies involving sleep deprived subjects’ performance on choice reaction time tests—in which response inhibition, task shifting skill and task strategy were involved—have been conducted and analyzed. These three cognitive processes are involved and critical in tasks involving supervisory attention, which is defined as behaviour that arises through the selection and implementation of schemas. [6] Following one night of total sleep deprivation, subjects showed no decline in task shifting or response inhibition performance. However, sleep deprivation does affect the ability to use preparatory bias to increase performance speed. It is suggested that the brain’s cognitive resources make an active effort to succeed in a challenging task when subjected to sleep deprivation, and that this deficit becomes apparent in tasks involving preparatory bias. [11] Similarly, partial sleep deprivation significantly influenced subjects simple reaction time, thus making it slower than subjects who were well rested [12]
Visuospatial attention
Deficits in cognitive performance due to continuous sleep restriction are not well understood. However, there have been studies looking into physiological arousal of the sleep-deprived brain. Participants, whose total amount of sleep had been restricted by 33% throughout one week, were subjected to reaction time tests. The results of these tests were analyzed using quantitative EEG analysis. The results indicate that the frontal regions of the brain are first to be affected, whereas the parietal regions remain active until the effects of sleep deprivation become more severe, which occurred towards the end of the week. In addition, EEG and ERP analysis reveals that activation deficits are more apparent in the non-dominant hemisphere—than in the dominant hemisphere. [13]
The effects of sleep deprivation on cognitive performance have been studied through the use of parametric visual attention tasks. Functional magnetic resonance imaging of participants' brains who were involved in ball-tracking tasks of various difficulty levels were obtained. These images were taken during rested wakefulness and again after one night of sleep deprivation. The thalamus is more highly activated when accompanied by sleep deprivation—than when the subject is in a state of rested wakefulness. Contrarily, the thalamus is more highly activated during difficult tasks accompanied by rested wakefulness, but not during a state of sleep deprivation. Researchers propose that the thalamic resources, which are normally activated during difficult tasks, are being activated in an attempt to maintain alertness during states of sleep deprivation. In addition, an increase in thalamic activation is related to a decrease in the parietal, prefrontal and cingulate cortex activation, resulting in the overall impairment of attentional networks, which are necessary for visuospatial attention performance. [14]
Functional Magnetic Resonance Imaging (fMRI) studies indicate that the posterior cingulate (PCC) and medial prefrontal cortex are involved in the anticipatory allocation of spatial attention. When sleep-deprived, PCC activity decreases, impairing selective attention. Subjects were exposed to an attention-shifting task involving spatially informative, misleading and uninformative cues preceding the stimuli. When sleep-deprived, subjects showed increased activation in the left intraparietal sulcus. This region is activated when exposed to stimuli in unexpected locations. These findings suggest that sleep-deprived people may be impaired in their ability to anticipate the locations of upcoming events. In addition, inability to avoid attending to irrelevant events may also be induced by sleep-deprivation. [15]
By contrast, other studies have indicated that the effects of sleep deprivation on cognitive performance, specifically sustained visual attention, are more global and bilateral in nature (as opposed to more lateralized deficit explanations). In a study using the Choice Visual Perception Task, subjects were exposed to stimuli appearing in various locations in visual space. Results indicate that sleep deprivation results in a general decline in visual attention. It is also suggested that the sleep-deprived brain is able to maintain a certain level of cognitive performance during tasks requiring divided attention—by recruiting additional cortical regions that are not normally used for such tasks. [16]