
Young's modulus is a mechanical property of solid materials that measures the tensile or compressive stiffness when the force is applied lengthwise. It is the modulus of elasticity for tension or axial compression. Young's modulus is defined as the ratio of the stress applied to the object and the resulting axial strain in the linear elastic region of the material.
The speed of sound in any chemical element in the fluid phase has one temperature-dependent value. In the solid phase, different types of sound wave may be propagated, each with its own speed: among these types of wave are longitudinal, transversal, and extensional.

Zirconium nitride is an inorganic compound used in a variety of ways due to its properties.

Tin telluride is a compound of tin and tellurium (SnTe); is a IV-VI narrow band gap semiconductor and has direct band gap of 0.18 eV. It is often alloyed with lead to make lead tin telluride, which is used as an infrared detector material.

Boron arsenide is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide B12As2. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects.
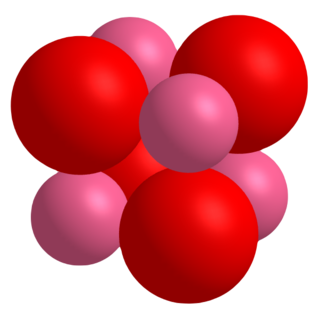
Cobalt(II) oxide is an inorganic compound that has been described as an olive-green or gray solid. It is used extensively in the ceramics industry as an additive to create blue-colored glazes and enamels, as well as in the chemical industry for producing cobalt(II) salts. A related material is cobalt(II,III) oxide, a black solid with the formula Co3O4.
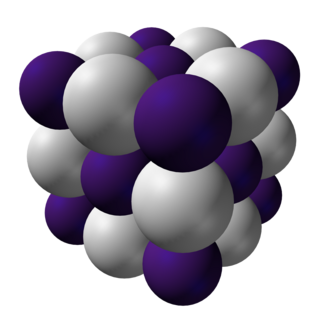
Caesium hydride or cesium hydride (CsH) is a compound of caesium and hydrogen. It is an alkali metal hydride. It was the first substance to be created by light-induced particle formation in metal vapor, and showed promise in early studies of an ion propulsion system using caesium. It is the most reactive stable alkaline metal hydride of all. It is a powerful superbase and reacts with water extremely vigorously.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.









