Applications

Electroless copper plating is used in the manufacture of printed circuit boards (PCBs), in particular for the conductive layer on the walls of through holes and vias.
Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde. [1]
Unlike electroplating, electroless plating processes in general not require passing an electric current through the bath and the substrate; the reduction of the metal cations in solution to metallic is achieved by purely chemical means, through an autocatalytic reaction. Thus electroless plating creates an even layer of metal regardless of the geometry of the surface – in contrast to electroplating which suffers from uneven current density due to the effect of substrate shape on the electric field at its surface. [2] Moreover, electroless plating can be applied to non-conductive surfaces.
In a typical formulation of the process, the surfaces to be coated are primed with a palladium catalyst and then immersed in a bath containing copper ions Cu2+, which are reduced by formaldehyde through the overall reactions[ citation needed ]

Electroless copper plating is used in the manufacture of printed circuit boards (PCBs), in particular for the conductive layer on the walls of through holes and vias.

Electrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. These reactions involve electric charges moving between electrodes and an electrolyte. Thus electrochemistry deals with the interaction between electrical energy and chemical change.
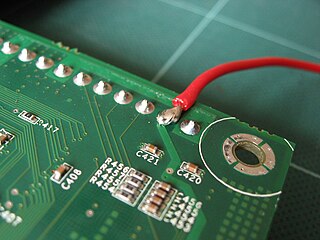
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to adhere to and connect the pieces after cooling, which requires that an alloy suitable for use as solder have a lower melting point than the pieces being joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.

A printed circuit board (PCB) mechanically supports and electrically connects electrical or electronic components using conductive tracks, pads and other features etched from one or more sheet layers of copper laminated onto and/or between sheet layers of a non-conductive substrate. Components are generally soldered onto the PCB to both electrically connect and mechanically fasten them to it.

Chrome plating, often referred to simply as chrome, is a technique of electroplating a thin layer of chromium onto a metal object. The chromed layer can be decorative, provide corrosion resistance, ease cleaning procedures, or increase surface hardness. Sometimes, a less expensive imitator of chrome may be used for aesthetic purposes.
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. Into a container of water are placed a copper rod, and the item. The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item. The copper rod is the anode and the item is the cathode. This current flow causes the copper to ionize, become oxidized which means each atom becomes positively charged by losing an electron. As the copper ions dissolve into the water, they form a coordination complex with salts already present. The copper then physically flows to the item, where it is reduced to the metallic state by gaining electrons. This forms a thin, solid, metallic copper film on the surface of the item.

Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver, by chemical or electrochemical plating. This article covers plating methods used in the modern electronics industry; for more traditional methods, often used for much larger objects, see gilding.

Metallizing is the general name for the technique of coating metal on the surface of objects. Metallic coatings may be decorative, protective or functional.

Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution via a process commonly referred to as leaching. Electrorefining uses a similar process to remove impurities from a metal. Both processes use electroplating on a large scale and are important techniques for the economical and straightforward purification of non-ferrous metals. The resulting metals are said to be electrowon.
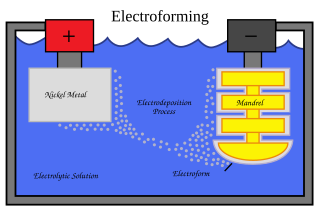
Electroforming is a metal forming process in which parts are fabricated through electrodeposition on a model, known in the industry as a mandrel. Conductive (metallic) mandrels are treated to create a mechanical parting layer, or are chemically passivated to limit electroform adhesion to the mandrel and thereby allow its subsequent separation. Non-conductive mandrels require the deposition of a conductive layer prior to electrodeposition. Such layers can be deposited chemically, or using vacuum deposition techniques. The outer surface of the mandrel forms the inner surface of the form.

Compact disc manufacturing is the process by which commercial compact discs (CDs) are replicated in mass quantities using a master version created from a source recording. This may be either in audio form (CD-Audio) or data form (CD-ROM). This process is used in the mastering of read-only compact discs. DVDs and Blu-rays use similar methods.

Electroless plating, also known as chemical plating or autocatalytic plating, is a class of industrial chemical processes that create metal coatings on various materials by autocatalytic chemical reduction of metal cations in a liquid bath. This class is contrasted with electroplating processes, such as galvanization, where the reduction is achieved by an externally generated electric current.

LIGA is a German acronym for Lithographie, Galvanoformung, Abformung that describes a fabrication technology used to create high-aspect-ratio microstructures.

Electroless nickel-phosphorus plating is a chemical process that deposits an even layer of nickel-phosphorus alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a phosphorus-containing reducing agent, usually a hypophosphite salt. It is the most common version of electroless nickel plating and is often referred just by that name. A similar process uses a borohydride reducing agent, yielding a nickel-boron coating instead.

Glass-to-metal seals are a very important element of the construction of vacuum tubes, electric discharge tubes, incandescent light bulbs, glass encapsulated semiconductor diodes, reed switches, pressure tight glass windows in metal cases, and metal or ceramic packages of electronic components.
Electroless nickel immersion gold (ENIG) is a metal plating process used in the manufacture of printed circuit boards (PCBs), to avoid oxidation and improve the solderability of copper contacts and plated through-holes. It consists of an electroless nickel plating covered with a thin layer of gold, which protects the nickel from oxidation. The gold is typically applied by quick immersion in a solution containing gold salts. Some of the nickel is oxidized to Ni2+ while the gold is reduced to metallic state. A variant of this process adds a thin layer of electroless palladium over the nickel, a process known by the acronym ENEPIG.
A molded interconnect device (MID) is an injection-molded thermoplastic part with integrated electronic circuit traces. The use of high temperature thermoplastics and their structured metallization opens a new dimension of circuit carrier design to the electronics industry. This technology combines plastic substrate/housing with circuitry into a single part by selective metallization.
Nickel electroplating is a technique of electroplating a thin layer of nickel onto a metal object. The nickel layer can be decorative, provide corrosion resistance, wear resistance, or used to build up worn or undersized parts for salvage purposes.

Electroless nickel-boron coating is a metal plating process that can create a layer of a nickel-boron alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a boron-containing reducing agent, such as an alkylamineborane or sodium borohydride. It is a type of electroless nickel plating. A similar process, that uses a hypophosphite as a reducing agent, yields a nickel-phosphorus coating instead.
| This metalworking article is a stub. You can help Wikipedia by expanding it. |