Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease and mouth disease.

Erythropoiesis is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.
Anemia of chronic disease (ACD) or anemia of chronic inflammation is a form of anemia seen in chronic infection, chronic immune activation, and malignancy. These conditions all produce elevation of interleukin-6, which stimulates hepcidin production and release from the liver. Hepcidin production and release shuts down ferroportin, a protein that controls export of iron from the gut and from iron storing cells. As a consequence, circulating iron levels are reduced. Other mechanisms may also play a role, such as reduced erythropoiesis. It is also known as anemia of inflammation, or anemia of inflammatory response.

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.

Hepcidin is a protein that in humans is encoded by the HAMP gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.

Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the SLC40A1 gene. Ferroportin is a transmembrane protein that transports iron from the inside of a cell to the outside of the cell. Ferroportin is the only known iron exporter.

GATA-binding factor 1 or GATA-1 is the founding member of the GATA family of transcription factors. This protein is widely expressed throughout vertebrate species. In humans and mice, it is encoded by the GATA1 and Gata1 genes, respectively. These genes are located on the X chromosome in both species.
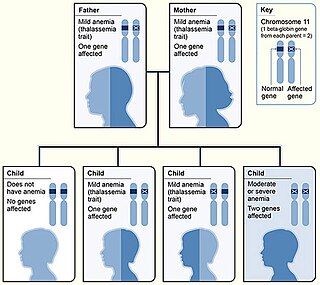
Beta thalassemias are a group of inherited blood disorders. They are forms of thalassemia caused by reduced or absent synthesis of the beta chains of hemoglobin that result in variable outcomes ranging from severe anemia to clinically asymptomatic individuals. Global annual incidence is estimated at one in 100,000. Beta thalassemias occur due to malfunctions in the hemoglobin subunit beta or HBB. The severity of the disease depends on the nature of the mutation.

Hephaestin, also known as HEPH, is a protein which in humans is encoded by the HEPH gene.

Hemojuvelin (HJV), also known as repulsive guidance molecule C (RGMc) or hemochromatosis type 2 protein (HFE2), is a membrane-bound and soluble protein in mammals that is responsible for the iron overload condition known as juvenile hemochromatosis in humans, a severe form of hemochromatosis. In humans, the hemojuvelin protein is encoded by the HFE2 gene. Hemojuvelin is a member of the repulsive guidance molecule family of proteins. Both RGMa and RGMb are found in the nervous system, while hemojuvelin is found in skeletal muscle and the liver.

Iron is an important biological element. It is used in both the ubiquitous iron-sulfur proteins and in vertebrates it is used in hemoglobin which is essential for blood and oxygen transport.

Krüppel-like Factor 2 (KLF2), also known as lung Krüppel-like Factor (LKLF), is a protein that in humans is encoded by the KLF2 gene on chromosome 19. It is in the Krüppel-like factor family of zinc finger transcription factors, and it has been implicated in a variety of biochemical processes in the human body, including lung development, embryonic erythropoiesis, epithelial integrity, T-cell viability, and adipogenesis.
Congenital hemolytic anemia (CHA) is a diverse group of rare hereditary conditions marked by decreased life expectancy and premature removal of erythrocytes from blood flow. Defects in erythrocyte membrane proteins and red cell enzyme metabolism, as well as changes at the level of erythrocyte precursors, lead to impaired bone marrow erythropoiesis. CAH is distinguished by variable anemia, chronic extravascular hemolysis, decreased erythrocyte life span, splenomegaly, jaundice, biliary lithiasis, and iron overload. Immune-mediated mechanisms may play a role in the pathogenesis of these uncommon diseases, despite the paucity of data regarding the immune system's involvement in CHAs.

Glutaredoxin 5, also known as GLRX5, is a protein which in humans is encoded by the GLRX5 gene located on chromosome 14. This gene encodes a mitochondrial protein, which is evolutionarily conserved. It is involved in the biogenesis of iron- sulfur clusters, which are required for normal iron homeostasis. Mutations in this gene are associated with autosomal recessive pyridoxine-refractory sideroblastic anemia.
Ineffective erythropoiesis is defined by the expansion of early-stage erythroid precursors driven by erythropoietin, accompanied by the apoptosis of late-stage precursors. This mechanism is principally responsible for the anemia seen in acquired conditions such as certain subtypes of myelodysplastic syndrome (MDS) and inherited disorders such as β-thalassemia, inherited sideroblastic anemias, as well as congenital dyserythropoietic anemias.
A myokine is one of several hundred cytokines or other small proteins and proteoglycan peptides that are produced and released by skeletal muscle cells in response to muscular contractions. They have autocrine, paracrine and/or endocrine effects; their systemic effects occur at picomolar concentrations.
Elizabeta Nemeth is an American physiologist who has made many contributions to the understanding of inflammatory disorders, thalassemias, and iron overload diseases.
Hemochromatosis type 4 is a hereditary iron overload disorder that affects ferroportin, an iron transport protein needed to export iron from cells into circulation. Although the disease is rare, it is found throughout the world and affects people from various ethnic groups. While the majority of individuals with type 4 hemochromatosis have a relatively mild form of the disease, some affected individuals have a more severe form. As the disease progresses, iron may accumulate in the tissues of affected individuals over time, potentially resulting in organ damage.

Adropin is a peptide encoded by the energy homeostasis-associated gene ENHO, which is highly conserved across mammals.
Hepatokines are proteins produced by liver cells (hepatocytes) that are secreted into the circulation and function as hormones across the organism. Research is mostly focused on hepatokines that play a role in the regulation of metabolic diseases such as diabetes and fatty liver and include: Adropin, ANGPTL4, Fetuin-A, Fetuin-B, FGF-21, Hepassocin, LECT2, RBP4,Selenoprotein P, Sex hormone-binding globulin.
















