Related Research Articles

The striatum, or corpus striatum, is a nucleus in the subcortical basal ganglia of the forebrain. The striatum is a critical component of the motor and reward systems; receives glutamatergic and dopaminergic inputs from different sources; and serves as the primary input to the rest of the basal ganglia.

Pavlovian fear conditioning is a behavioral paradigm in which organisms learn to predict aversive events. It is a form of learning in which an aversive stimulus is associated with a particular neutral context or neutral stimulus, resulting in the expression of fear responses to the originally neutral stimulus or context. This can be done by pairing the neutral stimulus with an aversive stimulus. Eventually, the neutral stimulus alone can elicit the state of fear. In the vocabulary of classical conditioning, the neutral stimulus or context is the "conditional stimulus" (CS), the aversive stimulus is the "unconditional stimulus" (US), and the fear is the "conditional response" (CR).

The nucleus accumbens is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia. The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle. Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
Wakefulness is a daily recurring brain state and state of consciousness in which an individual is conscious and engages in coherent cognitive and behavioral responses to the external world.

The periaqueductal gray is a brain region that plays a critical role in autonomic function, motivated behavior and behavioural responses to threatening stimuli. PAG is also the primary control center for descending pain modulation. It has enkephalin-producing cells that suppress pain.

The reticular formation is a set of interconnected nuclei that are located throughout the brainstem. It is not anatomically well defined, because it includes neurons located in different parts of the brain. The neurons of the reticular formation make up a complex set of networks in the core of the brainstem that extend from the upper part of the midbrain to the lower part of the medulla oblongata. The reticular formation includes ascending pathways to the cortex in the ascending reticular activating system (ARAS) and descending pathways to the spinal cord via the reticulospinal tracts.

The lateral hypothalamus (LH), also called the lateral hypothalamic area (LHA), contains the primary orexinergic nucleus within the hypothalamus that widely projects throughout the nervous system; this system of neurons mediates an array of cognitive and physical processes, such as promoting feeding behavior and arousal, reducing pain perception, and regulating body temperature, digestive functions, and blood pressure, among many others. Clinically significant disorders that involve dysfunctions of the orexinergic projection system include narcolepsy, motility disorders or functional gastrointestinal disorders involving visceral hypersensitivity, and eating disorders.

The reward system is a group of neural structures responsible for incentive salience, associative learning, and positively-valenced emotions, particularly ones involving pleasure as a core component. Reward is the attractive and motivational property of a stimulus that induces appetitive behavior, also known as approach behavior, and consummatory behavior. A rewarding stimulus has been described as "any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward". In operant conditioning, rewarding stimuli function as positive reinforcers; however, the converse statement also holds true: positive reinforcers are rewarding.

The medial dorsal nucleus is a large nucleus in the thalamus.
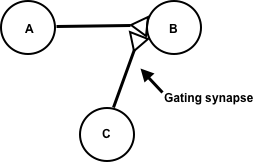
Synaptic gating is the ability of neural circuits to gate inputs by either suppressing or facilitating specific synaptic activity. Selective inhibition of certain synapses has been studied thoroughly, and recent studies have supported the existence of permissively gated synaptic transmission. In general, synaptic gating involves a mechanism of central control over neuronal output. It includes a sort of gatekeeper neuron, which has the ability to influence transmission of information to selected targets independently of the parts of the synapse upon which it exerts its action.
Coincidence detection in the context of neurobiology is a process by which a neuron or a neural circuit can encode information by detecting the occurrence of temporally close but spatially distributed input signals. Coincidence detectors influence neuronal information processing by reducing temporal jitter, reducing spontaneous activity, and forming associations between separate neural events. This concept has led to a greater understanding of neural processes and the formation of computational maps in the brain.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient.
Memory allocation is a process that determines which specific synapses and neurons in a neural network will store a given memory. Although multiple neurons can receive a stimulus, only a subset of the neurons will induce the necessary plasticity for memory encoding. The selection of this subset of neurons is termed neuronal allocation. Similarly, multiple synapses can be activated by a given set of inputs, but specific mechanisms determine which synapses actually go on to encode the memory, and this process is referred to as synaptic allocation. Memory allocation was first discovered in the lateral amygdala by Sheena Josselyn and colleagues in Alcino J. Silva's laboratory.
Many experiments have been done to find out how the brain interprets stimuli and how animals develop fear responses. The emotion, fear, has been hard-wired into almost every individual, due to its vital role in the survival of the individual. Researchers have found that fear is established unconsciously and that the amygdala is involved with fear conditioning.
Thomas S. Kilduff is an American neuroscientist and the director of SRI International's Center for Neuroscience. He specializes in neurobiology related to sleep and wakefulness, and was involved in the discovery of hypocretin, a neuropeptide system that is highly involved in wakefulness regulation.

Kay M. Tye is an American neuroscientist and professor and Wylie Vale Chair in the Salk Institute for Biological Sciences. Her research has focused on using optogenetics to identify connections in the brain that are involved in innate emotion, motivation and social behaviors.
Rosalind Anne Segal is an American neurobiologist. She is a Professor of Neurobiology at Harvard Medical School and the Co-Chair of the Cancer Biology Department at the Dana Farber Cancer Institute. Segal's work employs modern methods of cell and molecular biology to study the development of the mammalian brain with the goal of understanding how disruption of this normal process leads to the formation of brain malignancies.

Kafui Dzirasa is an American psychiatrist and Associate Professor at Duke University. He looks to understand the relationship between neural circuit malfunction and mental illness. He was a 2019 AAAS Leshner Fellow and was elected Fellow of the National Academy of Medicine in 2021.
Nadine Gogolla is a Research Group Leader at the Max Planck Institute of Neurobiology in Martinsried, Germany as well as an Associate Faculty of the Graduate School for Systemic Neuroscience. Gogolla investigates the neural circuits underlying emotion to understand how the brain integrates external cues, feeling states, and emotions to make calculated behavioral decisions. Gogolla is known for her discovery using machine learning and two-photon microscopy to classify mouse facial expressions into emotion-like categories and correlate these facial expressions with neural activity in the insular cortex.

Susana Q. Lima is a Portuguese neuroscientist and principal investigator at the Champalimaud Centre for the Unknown in Lisbon, Portugal. Her research studies neural mechanisms of sexual behavior and mate choice.
References
- 1 2 3 4 "Fan Wang". MIT McGovern Institute. Retrieved 2021-11-10.
- ↑ Wang, Fan (1998). Molecular genetic analysis of the olfactory sensory projections (PhD). Columbia University. OCLC 40523692 . Retrieved 2021-11-10.
- 1 2 3 Gustin, Georgina (August 24, 2021). "The pain switch". MIT Technology Review. Retrieved 2021-11-10.
- 1 2 "Fan Wang". Scholars@Duke. Duke University. Retrieved 2021-11-10.
- ↑ "Fan Wang". American Academy of Arts & Sciences. November 2021. Retrieved 2021-11-10.
- 1 2 Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD; et al. (2016). "Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit". Neuron. 92 (4): 739–753. doi:10.1016/j.neuron.2016.10.015. PMC 5172402 . PMID 27974160.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Tschida K, Michael V, Takatoh J, Han BX, Zhao S, Sakurai K; et al. (2019). "A Specialized Neural Circuit Gates Social Vocalizations in the Mouse". Neuron. 103 (3): 459–472.e4. doi:10.1016/j.neuron.2019.05.025. PMC 6687542 . PMID 31204083.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S; et al. (2017). "A craniofacial-specific monosynaptic circuit enables heightened affective pain". Nat Neurosci. 20 (12): 1734–1743. doi:10.1038/s41593-017-0012-1. PMC 5819335 . PMID 29184209.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Jiang-Xie LF, Yin L, Zhao S, Prevosto V, Han BX, Dzirasa K; et al. (2019). "A Common Neuroendocrine Substrate for Diverse General Anesthetics and Sleep". Neuron. 102 (5): 1053–1065.e4. doi:10.1016/j.neuron.2019.03.033. PMC 6554048 . PMID 31006556.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 "News Release: Brain Research Foundation Announces Recipients of 2016 Scientific Innovations Award" (PDF). Brain Research Foundation. February 11, 2016. Retrieved 2021-11-10.
- ↑ Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han BX; et al. (2020). "General anesthetics activate a potent central pain-suppression circuit in the amygdala". Nat Neurosci. 23 (7): 854–868. doi:10.1038/s41593-020-0632-8. PMC 7329612 . PMID 32424286.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Price, Kornbluth, and six senior faculty join American Academy of Arts & Sciences". Duke Today. Duke University. April 23, 2020. Retrieved 2021-11-10.
- ↑ "Fan Wang and Kafui Dzirasa receive $1 million award from Keck Foundation to research human consciousness". Duke Neurobiology. Duke University School of Medicine. August 10, 2016.
- ↑ "Five faculty named fellows of American Association for the Advancement of Science". Duke Today. Duke University. November 24, 2014. Retrieved 2021-11-10.
- ↑ "AAAS Fellow Class of 2014 Announced". American Association for the Advancement of Science. November 24, 2014. Retrieved 2021-11-10.
- ↑ "NIH Director's Pioneer Award Recipients". National Institutes of Health Office of Strategic Coordination - The Common Fund. July 23, 2021. Retrieved 2021-11-10.
- ↑ "2004 Annual Report" (PDF). Alfred P. Sloan Foundation. Retrieved 2021-11-10.