
The Grignard reaction is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides are added to the carbonyl groups of either an aldehyde or ketone under anhydrous conditions. This reaction is important for the formation of carbon–carbon bonds.
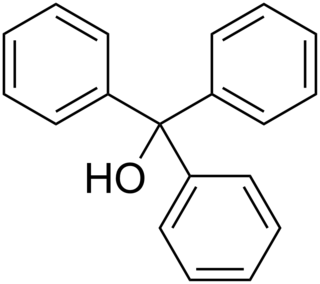
Triphenylmethanol is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation. Many derivatives of triphenylmethanol are important dyes.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Organocadmium chemistry describes the physical properties, synthesis, reactions, and use of organocadmium compounds, which are organometallic compounds containing a carbon to cadmium chemical bond. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited.

A Rieke metal is a highly reactive metal powder generated by reduction of a metal salt with an alkali metal. These materials are named after Reuben D. Rieke, who first described the recipes for their preparation. Among the many metals that have been generated by this method are Mg, Ca, Ti, Fe, Co, Ni, Cu, Zn, and In, which in turn are called Rieke-magnesium, Rieke-calcium, etc.
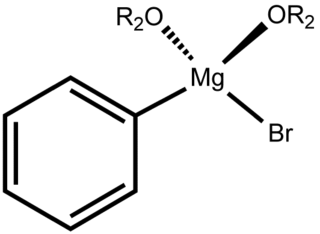
Phenylmagnesium bromide, with the simplified formula C
6H
5MgBr, is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is often used as a synthetic equivalent for the phenyl "Ph−" synthon.
A carbometallation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometallation.
The Kulinkovich reaction describes the organic synthesis of substituted cyclopropanols through reaction of esters with dialkyldialkoxytitanium reagents, which are generated in situ from Grignard reagents containing a hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was first reported by Oleg Kulinkovich and coworkers in 1989.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are rare and are typically limited to academic interests.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.

Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and imaging. Mesoporous ordered silica films have been also obtained with different pore topologies.
Methylmagnesium chloride is an organometallic compound with the general formula CH3MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.

Triphenylborane, often abbreviated to BPh3 where Ph is the phenyl group C6H5-, is a chemical compound with the formula B(C6H5)3. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents.
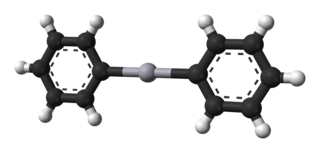
Diphenylmercury is the organomercury compound with the formula Hg(C6H5)2. It is a white solid. The compound is of historic interest as a particularly stable organometallic compound but it finds few uses because of its high toxicity.

tert-Butanesulfinamide is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are used in asymmetric synthesis as chiral auxiliaries, often as chiral ammonia equivalents for the synthesis of amines. tert-Butanesulfinamide and the associated synthetic methodology was introduced in 1997 by Jonathan A. Ellman et al.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.

Malika Jeffries-EL is an American chemist and professor of chemistry at Boston University studying organic semiconductors. Specifically, her research focuses on developing organic semiconductors that take advantage of the processing power of polymers and the electronic properties of semiconductors to create innovative electronic devices. She was elected as a Fellow of the American Chemical Society in 2018.

Fred Wudl is an American materials scientist, academic researcher. He is a Professor Emeritus in the Department of Materials Engineering at the University of California, Santa Barbara.
Kyoung-Shin Choi (Korean: 최경신) is a professor of chemistry at the University of Wisconsin-Madison. Choi's research focuses on the electrochemical synthesis of electrode materials, for use in electrochemical and photoelectrochemical devices.














