
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, gallium is in group 13 of the periodic table and is similar to the other metals of the group.

Glass is a non-crystalline solid that is often transparent, brittle and chemically inert. It has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.

Zone melting is a group of similar methods of purifying crystals, in which a narrow region of a crystal is melted, and this molten zone is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it as it moves through the ingot. The impurities concentrate in the melt, and are moved to one end of the ingot. Zone refining was invented by John Desmond Bernal and further developed by William G. Pfann in Bell Labs as a method to prepare high-purity materials, mainly semiconductors, for manufacturing transistors. Its first commercial use was in germanium, refined to one atom of impurity per ten billion, but the process can be extended to virtually any solute–solvent system having an appreciable concentration difference between solid and liquid phases at equilibrium. This process is also known as the float zone process, particularly in semiconductor materials processing.

Fused quartz,fused silica or quartz glass is a glass consisting of almost pure silica (silicon dioxide, SiO2) in amorphous (non-crystalline) form. This differs from all other commercial glasses in which other ingredients are added which change the glasses' optical and physical properties, such as lowering the melt temperature. Fused quartz, therefore, has high working and melting temperatures, making it less desirable for most common applications.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Arsenic trisulfide is the inorganic compound with the formula As2S3. It is a dark yellow solid that is insoluble in water. It also occurs as the mineral orpiment, which has been used as a pigment called King's yellow. It is produced in the analysis of arsenic compounds. It is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties.

Boron trioxide or diboron trioxide is the oxide of boron with the formula B2O3. It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.
Chalcogenide glass is a glass containing one or more chalcogens. Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.
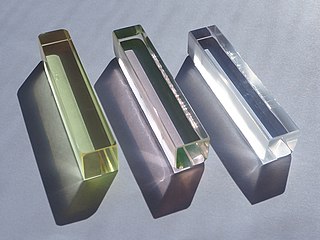
ZBLAN is the most stable, and consequently the most used, fluoride glass, a subcategory of the heavy metal fluoride glass (HMFG) group. Typically its composition is 53% ZrF4, 20% BaF2, 4% LaF3, 3% AlF3 and 20% NaF. ZBLAN is not a single material but rather has a spectrum of compositions, many of which are still untried. The biggest library in the world of ZBLAN glass compositions is currently owned by Le Verre Fluore, the oldest company working on HMFG technology. Other current ZBLAN fiber manufacturers are Thorlabs and KDD Fiberlabs. Hafnium fluoride is chemically similar to zirconium fluoride, and is sometimes used in place of it.

Lanthanum(III) oxide, also known as lanthana, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.
The indium chalcogenides include all compounds of indium with the chalcogen elements, oxygen, sulfur, selenium and tellurium. (Polonium is excluded as little is known about its compounds with indium). The best-characterised compounds are the In(III) and In(II) chalcogenides e.g. the sulfides In2S3 and InS.
This group of compounds has attracted a lot of research attention because they include semiconductors, photovoltaics and phase-change materials. In many applications indium chalcogenides are used as the basis of ternary and quaternary compounds such as indium tin oxide, ITO and copper indium gallium selenide, CIGS.
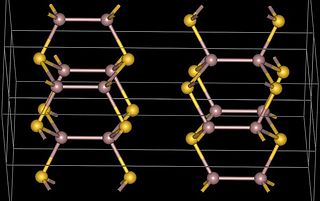
Gallium(II) sulfide, GaS, is a chemical compound of gallium and sulfur. The normal form of gallium(II) sulfide as made from the elements has a hexagonal layer structure containing Ga24+ units which have a Ga-Ga distance of 248pm. This layer structure is similar to GaTe, GaSe and InSe. An unusual metastable form, with a distorted wurtzite structure has been reported as being produced using MOCVD. The metal organic precursors were di-tert-butyl gallium dithiocarbamates, for example GatBu2(S2CNMe2) and this was deposited onto GaAs. The structure of the GaS produced in this way is presumably Ga2+ S2−.

Fluoride glass is a class of non-oxide optical glasses composed of fluorides of various metals. They can contain heavy metals such as zirconium, or be combined with lighter elements like aluminium and beryllium. These heavier elements cause the glass to have a transparency range extended into the infrared wavelength.
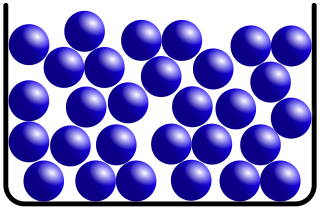
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials. Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions. Metallic glasses, for example, are typically well described by the dense random packing of hard spheres, whereas covalent systems, such as silicate glasses, have sparsely packed, strongly bound, tetrahedral network structures. These very different structures result in materials with very different physical properties and applications.
Chalcogenide chemical vapour deposition is a proposed technology for depositing thin films of chalcogenides, i.e. materials derived from sulfides, selenides, and tellurides. Conventional CVD can be used to deposit films of most metals, many non-metallic elements as well as a large number of compounds including carbides, nitrides, oxides. CVD can be used to synthesize chalcogenide glasses.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.

I-III-VI2 semiconductors are solid semiconducting materials that contain three or more chemical elements belonging to groups I, III and VI (IUPAC groups 1/11, 13 and 16) of the periodic table. They usually involve two metals and one chalcogen. Some of these materials have a direct bandgap, Eg, of approximately 1.5 eV, which makes them efficient absorbers of sunlight and thus potential solar cell materials. A fourth element is often added to a I-III-VI2 material to tune the bandgap for maximum solar cell efficiency. A representative example is copper indium gallium selenide (CuInxGa(1–x)Se2, Eg = 1.7–1.0 eV for x = 0–1), which is used in copper indium gallium selenide solar cells.
Tellurogallates are chemical compounds which contain anionic units of tellurium connected to gallium. They can be considered as gallates where tellurium substitutes for oxygen. Similar compounds include the thiogallates, selenogallates, telluroaluminates, telluroindates and thiostannates. They are in the category of chalcogenotrielates or more broadly tellurometallates or chalcogenometallates.
Optical glass refers to a quality of glass suitable for the manufacture of optical systems such as optical lenses, prisms or mirrors. Unlike window glass or crystal, whose formula is adapted to the desired aesthetic effect, optical glass contains additives designed to modify certain optical or mechanical properties of the glass: refractive index, dispersion, transmittance, thermal expansion and other parameters. Lenses produced for optical applications use a wide variety of materials, from silica and conventional borosilicates to elements such as germanium and fluorite, some of which are essential for glass transparency in areas other than the visible spectrum.















