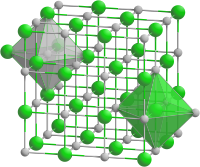
In chemistry, a phosphide is a compound containing the P3− ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides, which are solids consisting of anionic chains or clusters of phosphorus. Phosphides are known with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, and Po. Finally, some phosphides are molecular.
The nitridoborates are chemical compounds of boron and nitrogen with metals. These compounds are typically produced at high temperature by reacting hexagonal boron nitride with metal nitrides or by metathesis reactions involving nitridoborates. A wide range of these compounds have been made involving lithium, alkaline earth metals and lanthanides, and their structures determined using crystallographic techniques such as X-ray crystallography. Structurally one of their interesting features is the presence of polyatomic anions of boron and nitrogen where the geometry and the B–N bond length have been interpreted in terms of π-bonding.
The phosphidosilicates or phosphosilicides are inorganic compounds containing silicon bonded to phosphorus and one or more other kinds of elements. In the phosphosilicates each silicon atom is surrounded by four phosphorus atoms in a tetrahedron. The triphosphosilicates have a SiP3 unit, that can be a planar triangle like carbonate CO3. The phosphorus atoms can be shared to form different patterns e.g. [Si2P6]10− which forms pairs, and [Si3P7]3− which contains two-dimensional double layer sheets. [SiP4]8− with isolated tetrahedra, and [SiP2]2− with a three dimensional network with shared tetrahedron corners. SiP clusters can be joined, not only by sharing a P atom, but also by way of a P-P bond. This does not happen with nitridosilicates or plain silicates.
Hans Georg von Schnering was a German chemist and professor of inorganic chemistry at the University of Münster, honorary professor at the University of Stuttgart and director at the Max Planck Institute for Solid State Research.
The telluride phosphides are a class of mixed anion compounds containing both telluride and phosphide ions. The phosphidotelluride or telluridophosphide compounds have a [TeP]3− group in which the tellurium atom has a bond to the phosphorus atom. A formal charge of −2 is on the phosphorus and −1 on the tellurium. There is no binary compound of tellurium and phosphorus. Not many telluride phosphides are known, but they have been discovered for noble metals, actinides, and group 4 elements.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
A Phosphide chloride is a mixed anion compound containing both phosphide (P3−) and chloride (Cl−) ions.
Phosphide silicides or silicide phosphides or silicophosphides are compounds containing anions composed of silicide (Si4−) and phosphide (P3−). They can be considered as mixed anion compounds. They are distinct from the phosphidosilicates, which have the phosphorus bonded to the silicon. Related compounds include the phosphide carbides, germanide phosphides, nitride silicides, and antimonide silicides.
Phosphide carbides or carbide phosphides are compounds containing anions composed of carbide (C4−) and phosphide (P3−). They can be considered as mixed anion compounds. Related compounds include phosphide silicides, germanide phosphides, arsenide carbides, nitride carbides and silicide carbides.
Phosphanides are chemicals containing the [PH2]− anion. This is also known as the phosphino anion or phosphido ligand. The IUPAC name can also be dihydridophosphate(1−).
Lithium phosphide is an inorganic compound of lithium and phosphorus with the chemical formula Li3P. This dark colored compound is formally the lithium salt of phosphine, consisting of lithium cations Li+ and phosphide anions P3−. It is hazardous to handle because of its high reactivity toward air.
Phosphide iodides or iodide phosphides are compounds containing anions composed of iodide (I−) and phosphide (P3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the phosphide chlorides, arsenide iodides antimonide iodides and phosphide bromides.
Phosphide bromides or bromide phosphides are compounds containing anions composed of bromide (Br−) and phosphide (P3−) anions. Usually phosphorus is covalently connected into more complex structures. They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the phosphide chlorides, phosphide iodides, nitride bromides, arsenide bromides, and antimonide bromides.
Arsenide bromides or bromide arsenides are compounds containing anions composed of bromide (Br−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide chlorides, arsenide iodides, phosphide bromides, and antimonide bromides.
Arsenide iodides or iodide arsenides are compounds containing anions composed of iodide (I−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide chlorides, arsenide bromides, phosphide iodides, and antimonide iodides.
Antimonide bromides or bromide antimonides are compounds containing anions composed of bromide (Br−) and antimonide (Sb3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the antimonide chlorides, antimonide iodides, arsenide chlorides, arsenide bromides, arsenide iodides, phosphide chlorides, phosphide bromides, and phosphide iodides. The bromoantimonates have antimony in positive oxidation states.
Iodide hydrides are mixed anion compounds containing hydride and iodide anions. Many iodide hydrides are cluster compounds, containing a hydrogen atom in a core, surrounded by a layer of metal atoms, encased in a shell of iodide.
Phosphidogermanates are chemical compounds that have phosphorus bound to germanium to yield anions. They are in the category of phosphidotetrelates and also pnictides. They are analogous to nitridogermanates, phosphidoaluminates, phosphidogallates, phosphidoindates, phosphidosilicates or phosphidostannates.
Caesium phosphide refers to any of several inorganic compounds with the formula CsPx. The most studied member is Cs3P7, which forms yellow crystals of tetragonal structure (P41 group), which turn brown when heated to 300 °C, and colorless when cooled with liquid nitrogen.