Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.

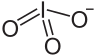
An iodate is the polyatomic anion with the formula IO−3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid.
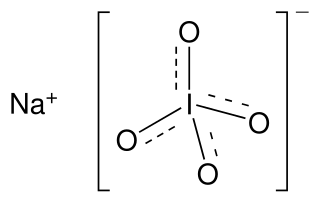
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.

Lanthanum(III) oxide, also known as lanthana, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.

Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses.
Calcium iodate is any of two inorganic compounds with the formula Ca(IO3)2(H2O)x, where x = 0 or 1. Both are colourless salts that occur as the minerals lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4. These minerals are the most common compounds containing iodate.
Lead(II) iodate is an inorganic compound with the molecular formula Pb(IO3)2. It is naturally found as heavy white powder.
The iodate fluorides are chemical compounds which contain both iodate and fluoride anions (IO3− and F−). In these compounds fluorine is not bound to iodine as it is in fluoroiodates.

Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+[(I−)2e−].
Iodate sulfates are mixed anion compounds that contain both iodate and sulfate anions. Iodate sulfates have been investigated as optical second harmonic generators, and for separation of rare earth elements. Related compounds include the iodate selenates and chromate iodates.
Praseodymium(III) iodate is an inorganic compound with the chemical formula Pr(IO3)3.
Neodymium(III) iodate is an inorganic compound with the chemical formula Nd(IO3)3.
Ytterbium(III) iodate is an inorganic compound with the chemical formula Yb(IO3)3. Its dihydrate can be prepared by reacting ytterbium sulfate and iodic acid in water at 200 °C. It crystallizes in the P21/c space group, with unit cell parameters a=8.685, b=6.066, c=16.687 Å, β=115.01°.
Promethium iodate is an inorganic compound with the chemical formula Pm(IO3)3. It can be obtained by reacting with potassium iodate, ammonium iodate or a slight excess of iodic acid and Pm3+ solution and precipitating it. Its hydrate, Pm(IO3)3·H2O, crystallizes in the P21 space group, with unit cell parameters a=10.172±13, b=6.700±20, c=7.289±24 Å, β=113.1±0.2°.
Terbium(III) iodate is an inorganic compound with the chemical formula Tb(IO3)3. It can be obtained by the reaction of terbium(III) periodate and periodic acid in water at 160 °C, or by the hydrothermal reaction of terbium(III) nitrate or terbium(III) chloride and iodic acid at 200 °C. It crystallizes in the monoclinic crystal system, with space group P21/c and unit cell parameters a=7.102, b=8.468, c=13.355 Å, β=99.67°.
Dysprosium iodate is an inorganic compound with the chemical formula Dy(IO3)3. It can be obtained by the reaction of dysprosium nitrate or dysprosium chloride and iodic acid at 200 °C. It exists in two crystal forms: α-form and β-form. Its solubility in water at 25 °C is 1.010±0.001 10−3 mol·dm−3). Adding ethanol or methanol to water will reduce the solubility.
Samarium iodate is an inorganic compound with the chemical formula Sm(IO3)3.
Europium(III) iodate is an inorganic compound with the chemical formula Eu(IO3)3. It can be produced by hydrothermal reaction of europium(III) nitrate or europium(III) oxide and iodic acid in water at 230 °C. It can be thermally decomposed as follows:
Zirconium iodate is an inorganic compound with the chemical formula Zr(IO3)4. It can be prepared by reacting sodium iodate and zirconium sulfate tetrahydrate in an aqueous solution. The resulting precipitate is dried and refluxed in concentrated nitric acid. Zirconium iodate trihydrate can be obtained by reacting hydrated zirconium oxide and iodine pentoxide (1.4~3.3% concentration) in water. Its basic salt Zr(OH)n(IO3)4−n is known.
Bismuth iodate is an inorganic compound with the chemical formula Bi(IO3)3. Its anhydrate can be obtained by reacting bismuth nitrate and iodic acid, dissolving the resulting precipitate in 7.8 mol/L nitric acid, and heating to volatilize and crystallize at 70 °C; The dihydrate can be obtained by reacting bismuth nitrate and potassium iodate or sodium iodate. It is obtained by evaporation and crystallization in 7 mol/L nitric acid at 50 °C. Its basic salt BiOIO3 is known.