
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O
2. Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.

The Proterozoic is the third of the four geologic eons of Earth's history, spanning the time interval from 2500 to 538.8 Mya, the longest eon of the Earth's geologic time scale. It is preceded by the Archean and followed by the Phanerozoic, and is the most recent part of the Precambrian "supereon".

The Paleoproterozoic Era is the first of the three sub-divisions (eras) of the Proterozoic eon, and also the longest era of the Earth's geological history, spanning from 2,500 to 1,600 million years ago (2.5–1.6 Ga). It is further subdivided into four geologic periods, namely the Siderian, Rhyacian, Orosirian and Statherian.
A reducing atmosphere is an atmospheric condition in which oxidation is prevented by absence of oxygen and other oxidizing gases or vapours, and which may contain actively reductant gases such as hydrogen, carbon monoxide, methane and hydrogen sulfide that would be readily oxidized to remove any free oxygen. Although Early Earth had had a reducing prebiotic atmosphere prior to the Proterozoic eon, starting at about 2.5 billion years ago in the late Neoarchaean period, the Earth's atmosphere experienced a significant rise in oxygen transitioned to an oxidizing atmosphere with a surplus of molecular oxygen (dioxygen, O2) as the primary oxidizing agent.

Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).
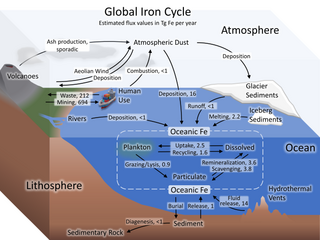
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

Geobiology is a field of scientific research that explores the interactions between the physical Earth and the biosphere. It is a relatively young field, and its borders are fluid. There is considerable overlap with the fields of ecology, evolutionary biology, microbiology, paleontology, and particularly soil science and biogeochemistry. Geobiology applies the principles and methods of biology, geology, and soil science to the study of the ancient history of the co-evolution of life and Earth as well as the role of life in the modern world. Geobiologic studies tend to be focused on microorganisms, and on the role that life plays in altering the chemical and physical environment of the pedosphere, which exists at the intersection of the lithosphere, atmosphere, hydrosphere and/or cryosphere. It differs from biogeochemistry in that the focus is on processes and organisms over space and time rather than on global chemical cycles.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

The Great Oxidation Event (GOE) or Great Oxygenation Event, also called the Oxygen Catastrophe, Oxygen Revolution, Oxygen Crisis or Oxygen Holocaust, was a time interval during the Early Earth's Paleoproterozoic Era when the Earth's atmosphere and the shallow ocean first experienced a rise in the concentration of oxygen. This began approximately 2.460–2.426 Ga (billion years) ago, during the Siderian period, and ended approximately 2.060 Ga, during the Rhyacian. Geological, isotopic, and chemical evidence suggests that biologically-produced molecular oxygen (dioxygen or O2) started to accumulate in Earth's atmosphere and changed it from a weakly reducing atmosphere practically devoid of oxygen into an oxidizing one containing abundant free oxygen, with oxygen levels being as high as 10% of their present atmospheric level by the end of the GOE.
Oxygenevolution is the process of generating molecular oxygen (O2) by a chemical reaction, usually from water. Oxygen evolution from water is effected by oxygenic photosynthesis, electrolysis of water, and thermal decomposition of various oxides. The biological process supports aerobic life. When relatively pure oxygen is required industrially, it is isolated by distilling liquefied air.
A paleoatmosphere is an atmosphere, particularly that of Earth, at some unspecified time in the geological past.
This article attempts to place key plant innovations in a geological context. It concerns itself only with novel adaptations and events that had a major ecological significance, not those that are of solely anthropological interest. The timeline displays a graphical representation of the adaptations; the text attempts to explain the nature and robustness of the evidence.

A microbial mat is a multi-layered sheet of microorganisms, mainly bacteria and archaea, or bacteria alone. Microbial mats grow at interfaces between different types of material, mostly on submerged or moist surfaces, but a few survive in deserts. A few are found as endosymbionts of animals.
The Pasteur point is a level of oxygen above which facultative aerobic microorganisms and facultative anaerobes adapt from fermentation to aerobic respiration. It is also used to mark the level of oxygen in the early atmosphere of the Earth that is believed to have led to major evolutionary changes. It is named after Louis Pasteur, the French microbiologist who studied anaerobic microbial fermentation, and is related to the Pasteur effect.

An autotroph is an organism that produces complex organic compounds using carbon from simple substances such as carbon dioxide, generally using energy from light (photosynthesis) or inorganic chemical reactions (chemosynthesis). They convert an abiotic source of energy into energy stored in organic compounds, which can be used by other organisms. Autotrophs do not need a living source of carbon or energy and are the producers in a food chain, such as plants on land or algae in water. Autotrophs can reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide.
The Boring Billion, otherwise known as the Mid Proterozoic and Earth's Middle Ages, is the time period between 1.8 and 0.8 billion years ago (Ga) spanning the middle Proterozoic eon, characterized by more or less tectonic stability, climatic stasis, and slow biological evolution. It is bordered by two different oxygenation and glacial events, but the Boring Billion itself had very low oxygen levels and no evidence of glaciation.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.

The prebiotic atmosphere is the second atmosphere present on Earth before today's biotic, oxygen-rich third atmosphere, and after the first atmosphere of Earth's formation. The formation of the Earth, roughly 4.5 billion years ago, involved multiple collisions and coalescence of planetary embryos. This was followed by a <100 million year period on Earth where a magma ocean was present, the atmosphere was mainly steam, and surface temperatures reached up to 8,000 K (14,000 °F). Earth's surface then cooled and the atmosphere stabilized, establishing the prebiotic atmosphere. The environmental conditions during this time period were quite different from today: the Sun was ~30% dimmer overall yet brighter at ultraviolet and x-ray wavelengths, there was a liquid ocean, it is unknown if there were continents but oceanic islands were likely, Earth's interior chemistry was different, and there was a larger flux of impactors hitting Earth's surface.

The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.
The Neoproterozoic Oxygenation Event (NOE), also called the Second Great Oxidation Event, was a time interval between around 850 and 540 million years ago which saw a very significant increase in oxygen levels in Earth's atmosphere and oceans. Bringing an end to the Boring Billion, a period of extremely low atmospheric oxygen spanning from the Statherian to the Tonian, the NOE was the second major increase in atmospheric and oceanic oxygen concentration on Earth, though it was not as major as the Great Oxidation Event (GOE) of the Neoarchean-Paleoproterozoic boundary. Unlike the GOE, it is unclear whether the NOE was a synchronous, global event or a series of asynchronous, regional oxygenation intervals with unrelated causes.












