
Staphylococcus aureus is a gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.
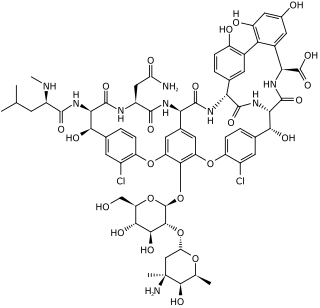
Vancomycin is a glycopeptide antibiotic medication used to treat certain bacterial infections. It is administered intravenously to treat complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally to treat Clostridioides difficile infections. When taken orally, it is poorly absorbed.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019.

Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.

Teicoplanin is an semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall peptidoglycan synthesis. It is used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis.

Vancomycin-resistant Staphylococcus aureus (VRSA) are strains of Staphylococcus aureus that have acquired resistance to the glycopeptide antibiotic vancomycin. Bacteria can acquire resistance genes either by random mutation or through the transfer of DNA from one bacterium to another. Resistance genes interfere with the normal antibiotic function and allow bacteria to grow in the presence of the antibiotic. Resistance in VRSA is conferred by the plasmid-mediated vanA gene and operon. Although VRSA infections are uncommon, VRSA is often resistant to other types of antibiotics and a potential threat to public health because treatment options are limited. VRSA is resistant to many of the standard drugs used to treat S. aureus infections. Furthermore, resistance can be transferred from one bacterium to another.

Dicloxacillin is a narrow-spectrum β-lactam antibiotic of the penicillin class. It is used to treat infections caused by susceptible (non-resistant) Gram-positive bacteria. It is active against beta-lactamase-producing organisms such as Staphylococcus aureus, which would otherwise be resistant to most penicillins. Dicloxacillin is available under a variety of trade names including Diclocil (BMS).

Oxacillin is a narrow-spectrum beta-lactam antibiotic of the penicillin class developed by Beecham.
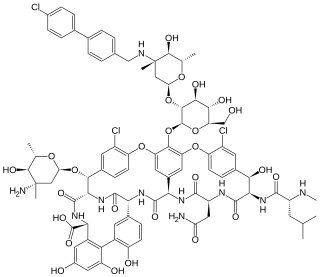
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.
Targanta Therapeutics Corporation was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company also had operations in Indianapolis, Montreal and Toronto. Targanta completed its initial public offering on October 9, 2007 and traded on the Nasdaq market under the symbol: TARG. Targanta was acquired by The Medicines Company in 2009.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).

Telavancin is a bactericidal lipoglycopeptide for use in MRSA or other Gram-positive infections. Telavancin is a semi-synthetic derivative of vancomycin.

Arbekacin (INN) is a semisynthetic aminoglycoside antibiotic which was derived from kanamycin. It is primarily used for the treatment of infections caused by multi-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Arbekacin was originally synthesized from dibekacin in 1973 by Hamao Umezawa and collaborators. It has been registered and marketed in Japan since 1990 under the trade name Habekacin. Arbekacin is no longer covered by patent and generic versions of the drug are also available under such trade names as Decontasin and Blubatosine.

Ceftaroline fosamil (INN), brand name Teflaro in the US and Zinforo in Europe, is a cephalosporin antibiotic with anti-MRSA activity. Ceftaroline fosamil is a prodrug of ceftaroline. It is active against methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive bacteria. It retains some activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria, but its effectiveness is relatively much weaker. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.

Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin.
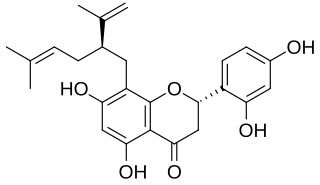
Sophoraflavanone G is a volatile phytoncide, released into the atmosphere, soil and ground water, by plants of the genus Sophora. Species include Sophora pachycarpa and Sophora exigua, all found to grow within the United States in a variety of soil types, within temperate conditions, no lower than 0 °F. Sophoraflavanone G is released in order to protect the plant against harmful protozoa, bacteria, and fungi. Sophoraflavanone G, also called kushenin, is a flavonoid compound.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.

Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics.
















