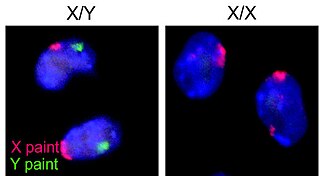
The XY sex-determination system is a sex-determination system used to classify many mammals, including humans, some insects (Drosophila), some snakes, some fish (guppies), and some plants. In this system, the sex of an individual is determined by a pair of sex chromosomes. In most cases, females have two of the same kind of sex chromosome (XX), and are called the homogametic sex. Males have two different kinds of sex chromosomes (XY), and are called the heterogametic sex.

A gonad, sex gland, or reproductive gland is a mixed gland that produces the gametes and sex hormones of an organism. Female reproductive cells are egg cells, and male reproductive cells are sperm. The male gonad, the testicle, produces sperm in the form of spermatozoa. The female gonad, the ovary, produces egg cells. Both of these gametes are haploid cells. Some hermaphroditic animals have a type of gonad called an ovotestis.

XY gonadal dysgenesis, also known as Swyer syndrome, is a type of defect hypogonadism in a person whose karyotype is 46,XY. Though they typically have normal vulvas, the person has underdeveloped gonads, fibrous tissue termed "streak gonads", and if left untreated, will not experience puberty. The cause is a lack or inactivation of an SRY gene which is responsible for sexual differentiation. Pregnancy is often possible in Swyer syndrome with assisted reproductive technology. The phenotype is usually similar to Turner syndrome (45,X0) due to a lack of X inactivation. The typical medical treatment is hormone replacement therapy. The syndrome was named after Gerald Swyer, an endocrinologist based in London.

The paramesonephric ducts are paired ducts of the embryo in the female reproductive system that run down the lateral sides of the genital ridge and terminate at the sinus tubercle in the primitive urogenital sinus. In the female, they will develop to form the fallopian tubes, uterus, cervix, and the upper one-third of the vagina.

Persistent Müllerian duct syndrome (PMDS) is the presence of Müllerian duct derivatives in what would be considered a genetically and otherwise physically normal male animal by typical human based standards. In humans, PMDS typically is due to an autosomal recessive congenital disorder and is considered by some to be a form of pseudohermaphroditism due to the presence of Müllerian derivatives.

Sex-determining region Y protein (SRY), or testis-determining factor (TDF), is a DNA-binding protein encoded by the SRY gene that is responsible for the initiation of male sex determination in therian mammals. SRY is an intronless sex-determining gene on the Y chromosome. Mutations in this gene lead to a range of disorders of sex development with varying effects on an individual's phenotype and genotype.

The male reproductive system consists of a number of sex organs that play a role in the process of human reproduction. These organs are located on the outside of the body, and within the pelvis.

XX male syndrome, also known as de la Chapelle syndrome, is a rare congenital intersex condition in which an individual with a 46,XX karyotype develops a male phenotype. Synonyms include 46,XX testicular difference of sex development, 46,XX sex reversal, nonsyndromic 46,XX testicular DSD, and XX sex reversal.

Ovotesticular syndrome is a term for an intersex condition in which an individual is born with both ovarian and testicular tissue. Commonly, one or both gonads is an ovotestis containing both types of tissue. Although it is similar in some ways to mixed gonadal dysgenesis, the conditions can be distinguished histologically.

Sex cords are embryonic structures which eventually will give rise (differentiate) to the adult gonads. They are formed from the genital ridges - which will develop into the gonads - in the first 2 months of gestation which depending on the sex of the embryo will give rise to male or female sex cords. These epithelial cells penetrate and invade the underlying mesenchyme to form the primitive sex cords. This occurs shortly before and during the arrival of the primordial germ cells (PGCs) to the paired genital ridges. If there is a Y chromosome present, testicular cords will develop via the Sry gene : repressing the female sex cord genes and activating the male. If there is no Y chromosome present the opposite will occur, developing ovarian cords. Prior to giving rise to sex cords, both XX and XY embryos have Müllerian ducts and Wolffian ducts. One of these structures will be repressed to induce the other to further differentiate into the external genitalia.
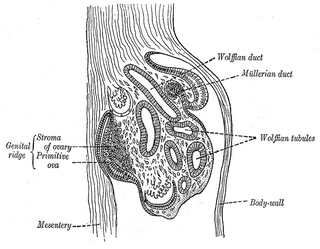
The genital ridge is the precursor to the gonads. The genital ridge initially consists mainly of mesenchyme and cells of underlying mesonephric origin. Once oogonia enter this area they attempt to associate with these somatic cells. Development proceeds and the oogonia become fully surrounded by a layer of cells.
XX gonadal dysgenesis is a type of female hypogonadism in which the ovaries do not function to induce puberty in an otherwise normal girl whose karyotype is found to be 46,XX. With nonfunctional streak ovaries, she is low in estrogen levels (hypoestrogenic) and has high levels of FSH and LH. Estrogen and progesterone therapy is usually then commenced. Some cases are considered a severe version of premature ovarian failure where the ovaries fail before puberty.

Sexual differentiation in humans is the process of development of sex differences in humans. It is defined as the development of phenotypic structures consequent to the action of hormones produced following gonadal determination. Sexual differentiation includes development of different genitalia and the internal genital tracts and body hair plays a role in sex identification.
Pseudohermaphroditism is a condition in which an individual has a matching chromosomal and gonadal tissue sex, but mismatching external genitalia.

Disorders of sex development (DSDs), also known as variations in sex characteristics (VSC), are congenital conditions affecting the reproductive system, in which development of chromosomal, gonadal, or anatomical sex is atypical.

The steroidogenic factor 1 (SF-1) protein is a transcription factor involved in sex determination by controlling the activity of genes related to the reproductive glands or gonads and adrenal glands. This protein is encoded by the NR5A1 gene, a member of the nuclear receptor subfamily, located on the long arm of chromosome 9 at position 33.3. It was originally identified as a regulator of genes encoding cytochrome P450 steroid hydroxylases, however, further roles in endocrine function have since been discovered.

WNT4 is a secreted protein that, in humans, is encoded by the WNT4 gene, found on chromosome 1. It promotes female sex development and represses male sex development. Loss of function may have consequences, such as female to male sex reversal.
46,XX/46,XY is a chimeric genetic condition characterized by the presence of some cells that express a 46,XX karyotype and some cells that express a 46,XY karyotype in a single human being. The cause of the condition lies in utero with the aggregation of two distinct blastocysts or zygotes into a single embryo, which subsequently leads to the development of a single individual with two distinct cell lines, instead of a pair of fraternal twins. 46,XX/46,XY chimeras are the result of the merging of two non-identical twins. This is not to be confused with mosaicism or hybridism, neither of which are chimeric conditions. Since individuals with the condition have two cell lines of the opposite sex, it can also be considered an intersex condition.
45,X/46,XY mosaicism, also known as X0/XY mosaicism and mixed gonadal dysgenesis, is a mutation of sex development in humans associated with sex chromosome aneuploidy and mosaicism of the Y chromosome. It is a fairly rare chromosomal disorder at birth, with an estimated incidence rate of about 1 in 15,000 live births. Mosaic loss of the Y chromosome in previously non-mosaic men grows increasingly common with age.
Sexual anomalies, also known as sexual abnormalities, are a set of clinical conditions due to chromosomal, gonadal and/or genitalia variation. Individuals with congenital (inborn) discrepancy between sex chromosome, gonadal, and their internal and external genitalia are categorised as individuals with a disorder of sex development (DSD). Afterwards, if the family or individual wishes, they can partake in different management and treatment options for their conditions.














