
Pfizer Inc. is an American multinational pharmaceutical and biotechnology corporation headquartered at The Spiral in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891).

Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half an hour and the duration is up to 36 hours.

F. Hoffmann-La Roche AG, commonly known as Roche, is a Swiss multinational holding healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange. The company headquarters are located in Basel. Roche is the fifth-largest pharmaceutical company in the world by revenue and the leading provider of cancer treatments globally. In 2023, the company’s seat in Forbes Global 2000 was 76.

The drug combination fenfluramine/phentermine, usually called fen-phen, was an anti-obesity treatment in the early 1990s that utilized two anorectics. Fenfluramine was marketed by American Home Products as Pondimin, but was shown to cause potentially fatal pulmonary hypertension and heart valve problems, which eventually led to its withdrawal in 1997 and legal damages of over $13 billion. Phentermine was not shown to have harmful effects.

Pulmonary hypertension is a condition of increased blood pressure in the arteries of the lungs. Symptoms include shortness of breath, fainting, tiredness, chest pain, swelling of the legs, and a fast heartbeat. The condition may make it difficult to exercise. Onset is typically gradual. According to the definition at the 6th World Symposium of Pulmonary Hypertension in 2018, a patient is deemed to have pulmonary hypertension if the pulmonary mean arterial pressure is greater than 20mmHg at rest, revised down from a purely arbitrary 25mmHg, and pulmonary vascular resistance (PVR) greater than 3 Wood units.

Bosentan, sold under the brand name Tracleer among others, is a dual endothelin receptor antagonist medication used in the treatment of pulmonary artery hypertension (PAH).

Iloprost, sold under the brand name Ventavis among others, is a medication used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud's phenomenon, frostbite, and other conditions in which the blood vessels are constricted and blood cannot flow to the tissues. Iloprost is a prostacyclin mimetic.

Nicox S.A. is a French ophthalmology company developing treatments to maintain vision and improve ocular health. Nicox is headquartered in Sophia Antipolis, France, and its Chairman and CEO is Michele Garufi.

Almorexant (INN), also known by its development code ACT-078573, is an orexin antagonist, acting as a competitive antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia. Development of the drug was abandoned in January 2011 due to concerns over the hepatic safety of almorexant after transient increases in liver enzymes were observed in trials.
Radium-223 is an isotope of radium with an 11.4-day half-life. It was discovered in 1905 by T. Godlewski, a Polish chemist from Kraków, and was historically known as actinium X (AcX). Radium-223 dichloride is an alpha particle-emitting radiotherapy drug that mimics calcium and forms complexes with hydroxyapatite at areas of increased bone turnover. The principal use of radium-223, as a radiopharmaceutical to treat metastatic cancers in bone, takes advantage of its chemical similarity to calcium, and the short range of the alpha radiation it emits.

Actelion Pharmaceuticals Ltd. is a pharmaceuticals and biotechnology company established in December 1997, headquartered in Allschwil near Basel, Switzerland.

United Therapeutics Corporation is an American publicly traded biotechnology company and public benefit corporation listed on the NASDAQ under the symbol UTHR. It develops novel, life-extending technologies for patients in the areas of lung disease and organ manufacturing. United Therapeutics is co-headquartered in Silver Spring, Maryland and Research Triangle Park, North Carolina, with additional facilities in Magog and Bromont, Quebec; Melbourne and Jacksonville, Florida; Blacksburg, Virginia; and Manchester, New Hampshire.
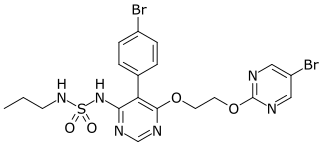
Macitentan, sold under the brand name Opsumit, is an endothelin receptor antagonist developed by Actelion and approved for the treatment of pulmonary arterial hypertension (PAH). Macitentan is a dual endothelin receptor antagonist, meaning that it acts as an antagonist of two endothelin (ET) receptor subtypes, ETA and ETB. However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype.

Selexipag, sold under the brand name Uptravi, is a medication developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679, are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation. It is taken by mouth or administered intravenously.
Alnylam Pharmaceuticals, Inc. is an American biopharmaceutical company focused on the discovery, development and commercialization of RNA interference (RNAi) therapeutics for genetically defined diseases. The company was founded in 2002 and is headquartered in Cambridge, Massachusetts. In 2016, Forbes included the company on its "100 Most Innovative Growth Companies" list.

Wilson Therapeutics is a biopharmaceutical company, based in Stockholm, Sweden, that develops novel therapies for rare diseases. The company is listed in the Mid-Cap segment on Nasdaq Stockholm with the stock ticker WTX.
Digital therapeutics, a subset of digital health, are evidence-based therapeutic interventions driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. Digital therapeutic companies should publish trial results inclusive of clinically meaningful outcomes in peer-reviewed journals. The treatment relies on behavioral and lifestyle changes usually spurred by a collection of digital impetuses. Because of the digital nature of the methodology, data can be collected and analyzed as both a progress report and a preventative measure. Treatments are being developed for the prevention and management of a wide variety of diseases and conditions, including type 1 & type II diabetes, congestive heart failure, obesity, Alzheimer's disease, dementia, asthma, substance abuse, ADHD, hypertension, anxiety, depression, and several others. Digital therapeutics often employ strategies rooted in cognitive behavioral therapy.
Roivant Sciences Ltd. is a healthcare company focused on applying technology to drug development and building subsidiary biotech and healthcare technology companies. It was founded in 2014 by Vivek Ramaswamy.
Abingworth LLP is a transatlantic bioscience investment firm founded in 1973 by a pair of London stockbrokers, Peter Dicks and Hon. Anthony Montagu. Abingworth had initially sought to extend its investments into the biotechnology industry in the late 1980s, and has subsequently been investing in life science and healthcare services companies since at least 2001, and had expanded to include information technology firm investments by 2016.
Sotatercept, sold under the brand name Winrevair is a medication used for the treatment of pulmonary arterial hypertension. It is an activin signaling inhibitor, based on the extracellular domain of the activin type 2 receptor expressed as a recombinant fusion protein with immunoglobulin Fc domain (ACTRIIA-Fc). It is given by subcutaneous injection.














