
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.

Hydroperoxides or peroxols are compounds of the form ROOH, where R stands for any group, typically organic, which contain the hydroperoxy functional group. Hydroperoxide also refers to the hydroperoxide anion and its salts, and the neutral hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.

tert-Butylphosphaacetylene is an organophosphorus compound. Abbreviated t-BuCP, it was the first example of an isolable phosphaalkyne. Prior to its synthesis, the double bond rule had suggested that elements of Period 3 and higher were unable to form double or triple bonds with lighter main group elements because of weak orbital overlap. The synthesis of t-BuCP discredited much of the double bond rule and opened new studies into the formation of unsaturated phosphorus compounds.
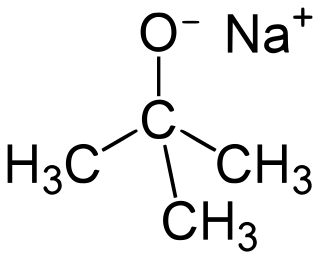
Sodium tert-butoxide (or sodium t-butoxide) is a chemical compound with the formula (CH3)3CONa (abbr. NaOtBu). It is a strong, non-nucleophilic base. It is flammable and moisture sensitive. It is sometimes written in the chemical literature as sodium t-butoxide. It is similar in reactivity to the more common potassium tert-butoxide.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.
Tungsten(IV) chloride is an inorganic compound with the formula WCl4. It is a diamagnetic black solid. The compound is of interest in research as one of a handful of binary tungsten chlorides.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
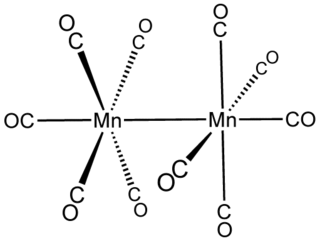
In inorganic chemistry, metal–metal bonds describe attractive interactions between metal centers. The simplest examples are found in bimetallic complexes. Metal–metal bonds can be "supported", i.e. be accompanied by one or more bridging ligands, or "unsupported". They can also vary according to bond order. The topic of metal–metal bonding is usually discussed within the framework of coordination chemistry, but the topic is related to extended metallic bonding, which describes interactions between metals in extended solids such as bulk metals and metal subhalides.

Hexa(tert-butoxy)dimolybdenum(III) is a coordination complex of molybdenum(III). It is one of the homoleptic alkoxides of molybdenum. An orange, air-sensitive solid, the complex has attracted academic attention as the precursor to many organomolybdenum derivatives. It an example of a charge-neutral complex featuring a molybdenum to molybdenum triple bond (Mo≡Mo), arising from the coupling of a pair of d3 metal centers. It can be prepared by a salt metathesis reaction from the THF complex of molybdenum trichloride and lithium tert-butoxide:

Lithium tert-butoxide is the metalorganic compound with the formula LiOC(CH3)3. A white solid, it is used as a strong base in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but it is not ionized in solution. Both octameric and hexameric forms have been characterized by X-ray crystallography
1-Phosphaallenes is are allenes in which the first carbon atom is replaced by phosphorus, resulting in the structure: -P=C=C<.
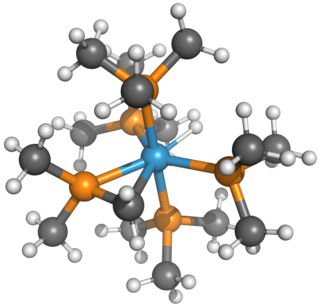
Pentakis(trimethylphosphine)tungsten (W(PMe3)5, Me=CH3) and its physically relevant "tucked-in" isomer, [(dimethylphosphino-κP)methyl-κC]hydrotetrakis(trimethylphosphine)tungsten (W(PMe3)4(η2-CH2PMe2)H), are organotungsten complexes. Formally, the former is a tungsten(0) complex, whereas the latter is a tungsten(II) complex. W(PMe3)4(η2-CH2PMe2)H's tungsten center is electron-rich and, thus, prone to oxidation. W(PMe3)4(η2-CH2PMe2)H has been used as a starting retron for some challenging chemistry, such as C-C bond activation, tungsten-chalcogenide multiple bonding, tungsten-tetrel multiple bonding, and desulfurization.























