
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.

Sir Geoffrey Wilkinson FRS was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have also been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.

Robert Howard GrubbsForMemRS was an American chemist and the Victor and Elizabeth Atkins Professor of Chemistry at the California Institute of Technology in Pasadena, California. He was a co-recipient of the 2005 Nobel Prize in Chemistry for his work on olefin metathesis.

Yves Chauvin was a French chemist and Nobel Prize laureate. He was honorary research director at the Institut français du pétrole and a member of the French Academy of Science. He was known for his work for deciphering the process of olefin metathesis for which he was awarded the 2005 Nobel Prize in Chemistry along with Robert H. Grubbs and Richard R. Schrock.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India (Hyderabad). In January, 2016 a major layoff marked the end of the organization.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.
Frederick Nye Tebbe was a chemist known for his work on organometallic chemistry. Tebbe was born in Oakland, California on March 20, 1935. His father, Charles L. Tebbe, worked for the United States Forest Service so Fred’s early education took place in Montana, Oregon, Maryland and Pennsylvania. He married Margaret Manzer in 1960, and they had a son and a daughter. He died of pancreatic cancer at his home in Delaware on September 28, 1995.

Herbert S. Eleuterio was an American industrial chemist noted for technical contributions to catalysis, polymerization, industrial research management, and science education. In particular, he discovered the olefin metathesis reaction and several novel fluoropolymers. Additionally, he explored techniques for research leadership, especially methods for fostering collaboration, globalization, and scientific creativity.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
T. Don Tilley is a professor of chemistry at the University of California, Berkeley.
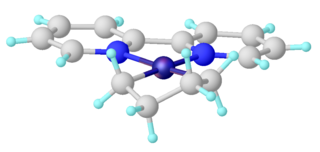
In organometallic chemistry, metallacyclopentanes are compounds with the formula LnM(CH2)4 (Ln = ligands, and M = metal). They are a type of metallacycle. Metallacyclopentanes are intermediates in some metal-catalysed reactions in homogeneous catalysis.
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.
Dr. Daniel Mindiola, a Venezuelan chemist, is the Brush Family Professor of Chemistry at the University of Pennsylvania. He specializes in inorganic and organometallic synthesis, catalysis, and mechanistic chemistry. He has over 200 peer-reviewed scientific publications.













