Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides.

Karl Barry Sharpless is an American chemist. He is a two-time Nobel laureate in Chemistry known for his work on stereoselective reactions and click chemistry.
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include:
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.
Chemical biology is a scientific discipline between the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.
In chemical synthesis, click chemistry is a class of simple, atom-economy reactions commonly used for joining two molecular entities of choice. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, biomimetic and molecular machinery applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".

Tris( methyl)amine (TBTA) is a tertiary amine containing the 1,2,3-triazole moiety. When used as a ligand, complexed to copper(I), it allows for quantitative, regioselective formal Huisgen 1,3-dipolar cycloadditions between alkynes and azides, in a variety of aqueous and organic solvents.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.
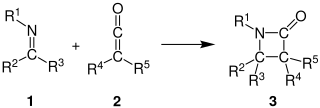
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

3-Azidocoumarin is an organic compound that is used in the area of bioconjugation. It is a derivative of coumarin, a natural product and precursor for the widely used Coumadin. Azidocoumarin has emerged as a widely applicable labeling agent in diverse biological systems. In particular, it participates in the aptly named click reaction with alkynes. Bioconjugation involves the labeling of certain cellular components and is applicable to fields such a proteomics and functional genomics with a detachable, fluorescent tag.
1-Decyne is the organic compound with the formula C8H17C≡CH. It is a terminal alkyne. A colorless liquid, 1-decyne is used as a model substrate when evaluating methodology in organic synthesis. It participates in a number of classical reactions including Suzuki-Miyaura couplings, Sonogashira couplings, Huisgen cycloadditions, and borylations.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.
A metal-centered cycloaddition is a subtype of the more general class of cycloaddition reactions. In such reactions "two or more unsaturated molecules unite directly to form a ring", incorporating a metal bonded to one or more of the molecules. Cycloadditions involving metal centers are a staple of organic and organometallic chemistry, and are involved in many industrially-valuable synthetic processes.
Clicked peptide polymers are poly-triazole-poly-peptide hybrid polymers. They are made of repeating units of a 1,2,3-triazole and an oligopeptide. They can be visualized as an oligopeptide that is flanked at both the C-terminus and N-terminus by a triazole molecule.
M. G. Finn is an American chemist and professor at the Georgia Institute of Technology.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.






