| |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | <10 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
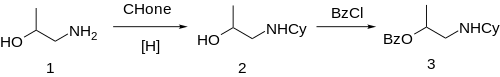
Hexylcaine hydrochloride, also called cyclaine (Merck) or osmocaine, is a short-acting local anesthetic. It acts by inhibiting sodium channel conduction. Overdose can lead to headache, tinnitus, numbness and tingling around the mouth and tongue, convulsions, inability to breathe, and decreased heart function. [1]