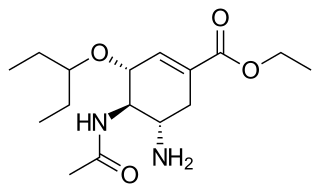
| NH winter season | Influenza A H1N1 | Influenza A H3N2 | Influenza B Victoria | Influenza B Yamagata |
|---|
| 1998–1999 [6] | A/Beijing/262/95 (H1N1)-like virus | A/Sydney/5/97 (H3N2)-like virus | N/A | B/Beijing/184/93-like virus |
| 1999–2000 [7] | A/Beijing/262/95 (H1N1)-like virus | A/Sydney/5/97 (H3N2)-like virus | B/Shangdong/7/97-like virus [note 1] | B/Beijing/184/93-like virus [note 1] |
| 2000–2001 [8] | A/New Caledonia/20/99 (H1N1)-like virus | A/Moscow/10/99 (H3N2)-like virus | N/A | B/Beijing/184/93-like virus |
| 2001–2002 [9] | A/New Caledonia/20/99 (H1N1)-like virus | A/Moscow/10/99 (H3N2)-like virus | N/A | B/Sichuan/379/99-like virus |
| 2002–2003 [10] | A/New Caledonia/20/99 (H1N1)-like virus | A/Moscow/10/99 (H3N2)-like virus | B/Hong Kong/330/2001-like virus | N/A |
| 2003–2004 [11] | A/New Caledonia/20/99 (H1N1)-like virus | A/Moscow/10/99 (H3N2)-like virus | B/Hong Kong/330/2001-like virus | N/A |
| 2004–2005 [12] | A/New Caledonia/20/99 (H1N1)-like virus | A/Fujian/411/2002 (H3N2)-like virus | N/A | B/Shanghai/361/2002-like virus |
| 2005–2006 [13] | A/New Caledonia/20/99 (H1N1)-like virus | A/California/7/2004 (H3N2)-like virus | N/A | B/Shanghai/361/2002-like virus |
| 2006–2007 [14] | A/New Caledonia/20/99 (H1N1)-like virus | A/Wisconsin/67/2005 (H3N2)-like virus | B/Malaysia/2506/2004-like virus | N/A |
| 2007–2008 [15] | A/Solomon Islands/3/2006 (H1N1)-like virus | A/Wisconsin/67/2005 (H3N2)-like virus | B/Malaysia/2506/2004-like virus | N/A |
| 2008–2009 [16] | A/Brisbane/59/2007 (H1N1)-like virus | A/Brisbane/10/2007 (H3N2)-like virus | N/A | B/Florida/4/2006-like virus |
| 2009–2010 [17] | A/Brisbane/59/2007 (H1N1)-like virus | A/Brisbane/10/2007 (H3N2)-like virus | B/Brisbane/60/2008-like virus | N/A |
| 2010–2011 [18] | A/California/7/2009 (H1N1)-like virus | A/Perth/16/2009 (H3N2)-like virus | B/Brisbane/60/2008-like virus | N/A |
| 2011–2012 [19] | A/California/7/2009 (H1N1)-like virus | A/Perth/16/2009 (H3N2)-like virus | B/Brisbane/60/2008-like virus | N/A |
| 2012–2013 [20] | A/California/7/2009 (H1N1)pdm09 [note 2] -like virus [21] | A/Victoria/361/2011 (H3N2)-like virus | B/Brisbane/60/2008-like virus | B/Wisconsin/1/2010-like virus |
| 2013–2014 [22] | A/California/7/2009 (H1N1)pdm09-like virus | A(H3N2) virus antigenically like the cell-propagated prototype virus A/Victoria/361/2011 [note 3] | B/Brisbane/60/2008-like virus | B/Massachusetts/2/2012-like virus |
| 2014–2015 [23] | A/California/7/2009 (H1N1)pdm09-like virus | A/Texas/50/2012 (H3N2)-like virus [note 4] | B/Brisbane/60/2008-like virus | B/Massachusetts/2/2012-like virus |
| 2015–2016 [24] | A/California/7/2009 (H1N1)pdm09-like virus | A/Switzerland/9715293/2013 (H3N2)-like virus | B/Brisbane/60/2008-like virus | B/Phuket/3073/2013-like virus |
| 2016–2017 [25] [26] | A/California/7/2009 (H1N1)pdm09-like virus | A/Hong Kong/4801/2014 (H3N2)-like virus | B/Brisbane/60/2008-like virus | B/Phuket/3073/2013-like virus |
| 2017–2018 [27] [28] | A/Michigan/45/2015 (H1N1)pdm09-like virus [29] | A/Hong Kong/4801/2014 (H3N2)-like virus | B/Brisbane/60/2008-like virus | B/Phuket/3073/2013-like virus |
| 2018–2019 [30] [31] | A/Michigan/45/2015 (H1N1)pdm09-like virus | A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus | B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) | B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) |
| 2019–2020 [32] [33] [34] | A/Brisbane/02/2018 (H1N1)pdm09-like virus | A/Kansas/14/2017 (H3N2)-like virus | B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) | B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) |
| 2020–2021 egg-based vaccines [35] | A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09-like virus | A/Hong Kong/2671/2019 (H3N2)-like virus | B/Washington/02/2019 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2020–2021 cell- or recombinant-based vaccines [35] | A/Hawaii/70/2019 (H1N1)pdm09-like virus | A/Hong Kong/45/2019 (H3N2)-like virus | B/Washington/02/2019 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2021–2022 egg-based vaccines [36] | A/Victoria/2570/2019 (H1N1)pdm09-like virus | A/Cambodia/e0826360/2020 (H3N2)-like virus | B/Washington/02/2019 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2021–2022 cell- or recombinant-based vaccines [36] | A/Wisconsin/588/2019 (H1N1)pdm09-like virus | A/Cambodia/e0826360/2020 (H3N2)-like virus | B/Washington/02/2019 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2022–2023 egg-based vaccines [37] | A/Victoria/2570/2019 (H1N1)pdm09-like virus | A/Darwin/9/2021 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2022–2023 cell- or recombinant-based vaccines [37] | A/Wisconsin/588/2019 (H1N1)pdm09-like virus | A/Darwin/6/2021 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2023–2024 egg-based vaccines [38] [39] | A/Victoria/4897/2022 (H1N1)pdm09-like virus | A/Darwin/9/2021 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2023–2024 cell- or recombinant-based vaccines [38] [39] | A/Wisconsin/67/2022 (H1N1)pdm09-like virus | A/Darwin/6/2021 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
| 2024–2025 egg-based vaccines [40] [5] | A/Victoria/4897/2022 (H1N1)pdm09-like virus | A/Thailand/8/2022 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus [note 5] |
| 2024–2025 cell- or recombinant-based vaccines [40] [5] | A/Wisconsin/67/2022 (H1N1)pdm09-like virus | A/Massachusetts/18/2022 (H3N2)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus [note 5] |
|