 | |
| Names | |
|---|---|
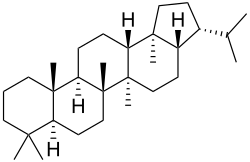
| IUPAC name Hopane [2] | |
| Systematic IUPAC name (3R,3aS,5aR,5bR,7aS,11aS,11bR,13aR,13bS)-5a,5b,8,8,11a,13b-Hexamethyl-3-(propan-2-yl)icosahydro-1H-cyclopenta[a]chrysene | |
| Other names A'-Neogammacerane | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C30H52 | |
| Molar mass | 412.746 g·mol−1 |
| Density | 0.952 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Hopane is a natural chemical compound classified as a triterpene. It forms the central core of a variety of other chemical compounds which are collectively known as hopanoids. The first compound of the hopane family to be isolated and characterised was hydroxyhopanone, found in dammar resin. [3] The name derives from Hopea , a tree genus from which dammar is obtained. [4]