Related Research Articles
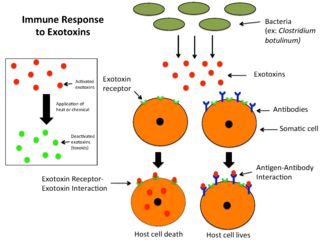
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

Hairy cell leukemia is an uncommon hematological malignancy characterized by an accumulation of abnormal B lymphocytes. It is usually classified as a subtype of chronic lymphocytic leukemia (CLL). Hairy cell leukemia makes up about 2% of all leukemias, with fewer than 2,000 new cases diagnosed annually in North America and Western Europe combined.
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow injection into a vein. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

A single-domain antibody (sdAb), also known as a NANOBODY®, is an antibody fragment consisting of a single monomeric variable antibody domain. Like a whole antibody, it is able to bind selectively to a specific antigen. With a molecular weight of only 12–15 kDa, single-domain antibodies are much smaller than common antibodies which are composed of two heavy protein chains and two light chains, and even smaller than Fab fragments and single-chain variable fragments.

Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell. It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies are used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Naptumomab estafenatox (ABR-217620) is a drug being developed for the treatment of various types of cancer like non-small cell lung carcinoma and renal cell carcinoma.

Ira Pastan is an American scientist at the National Cancer Institute. He is a member of the National Academy of Sciences, a Fellow of the AAAS and the American Society of Microbiology. In 2009, he was awarded the prestigious International Antonio Feltrinelli Prize for Medicine. His wife, Linda Pastan, was an American poet.

B-cell prolymphocytic leukemia, referred to as B-PLL, is a rare blood cancer. It is a more aggressive, but still treatable, form of leukemia.
An anti-CD22 immunotoxin is a monoclonal antibody linked to a cytotoxic agent. They are being studied in the treatment of some types of B-cell cancer.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Moxetumomab pasudotox, sold under the brand name Lumoxiti, is an anti-CD22 immunotoxin medication for the treatment of adults with relapsed or refractory hairy cell leukemia (HCL) who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog. Moxetumomab pasudotox is a CD22-directed cytotoxin and is the first of this type of treatment for adults with HCL. The drug consists of the binding fragment (Fv) of an anti-CD22 antibody fused to a toxin called PE38. This toxin is a 38 kDa fragment of Pseudomonas exotoxin A.
Resimmune or A-dmDT390-bisFv(UCHT1) is an experimental drug — an anti-T cell immunotoxin — that is being investigated for the treatment of T cell blood cancers such as cutaneous T cell lymphoma (CTCL). It was developed by Doctors Neville, Woo, and Liu while at the National Institutes of Health (NIH) and is under exclusive license to Angimmune, LLC. The therapy has potential applications for lymphomas and T cell driven autoimmune diseases, including multiple sclerosis, and graft-versus-host disease following stem cell or bone marrow transplant.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.
Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
References
- 1 2 Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ (2007). "Immunotoxin treatment of cancer". Annu. Rev. Med. 58: 221–37. doi:10.1146/annurev.med.58.070605.115320. PMID 17059365.
- ↑ Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ (July 2006). "Immunotoxin therapy of cancer". Nat. Rev. Cancer. 6 (7): 559–65. doi:10.1038/nrc1891. PMID 16794638.
- ↑ Pastan I, Ho M (2010). "Recombinant immunotoxins for treating cancer.". In Kontermann R, Dübel S (eds.). Antibody Engineering. Berlin, Heidelberg: Springer. pp. 127–146. doi:10.1007/978-3-642-01147-4_10. ISBN 978-3-642-01146-7.
- ↑ Kreitman RJ, Wilson WH, Bergeron K, et al. (July 2001). "Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia". N. Engl. J. Med. 345 (4): 241–7. doi: 10.1056/NEJM200107263450402 . PMID 11474661.
- ↑ Angimmune: Clinical Trials: Identification of a Cutaneous T-Cell Lymphoma (CTCL) Subgroup Experiencing a High Treatment Response Rate: Paragraph 1