The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the isthmus (pl.: isthmi). The thyroid gland is a butterfly-shaped gland located in the neck below the Adam's apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones – triiodothyronine (T3) and thyroxine (T4) – and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis and growth and development in children. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.
Hypothyroidism is a disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormones. It can cause a number of symptoms, such as poor ability to tolerate cold, a feeling of tiredness, constipation, slow heart rate, depression, and weight gain. Occasionally there may be swelling of the front part of the neck due to goitre. Untreated cases of hypothyroidism during pregnancy can lead to delays in growth and intellectual development in the baby or congenital iodine deficiency syndrome.

Iodothyronine deiodinases (EC 1.21.99.4 and EC 1.21.99.3) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor, influences the expression of genes in practically every vertebrate cell. Iodothyronine deiodinases are unusual in that these enzymes contain selenium, in the form of an otherwise rare amino acid selenocysteine.
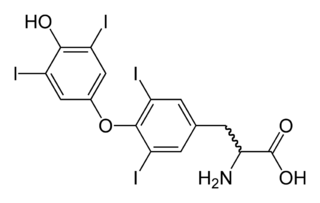
Triiodothyronine, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate.

Levothyroxine, also known as L-thyroxine, is a synthetic form of the thyroid hormone thyroxine (T4). It is used to treat thyroid hormone deficiency (hypothyroidism), including a severe form known as myxedema coma. It may also be used to treat and prevent certain types of thyroid tumors. It is not indicated for weight loss. Levothyroxine is taken orally (by mouth) or given by intravenous injection. Levothyroxine has a half-life of 7.5 days when taken daily, so about six weeks is required for it to reach a steady level in the blood.

Propylthiouracil (PTU) is a medication used to treat hyperthyroidism. This includes hyperthyroidism due to Graves' disease and toxic multinodular goiter. In a thyrotoxic crisis it is generally more effective than methimazole. Otherwise it is typically only used when methimazole, surgery, and radioactive iodine is not possible. It is taken by mouth.

Thyroid peroxidase, also called thyroperoxidase (TPO), thyroid specific peroxidase or iodide peroxidase, is an enzyme expressed mainly in the thyroid where it is secreted into colloid. Thyroid peroxidase oxidizes iodide ions to form iodine atoms for addition onto tyrosine residues on thyroglobulin for the production of thyroxine (T4) or triiodothyronine (T3), the thyroid hormones. In humans, thyroperoxidase is encoded by the TPO gene.

Thyroxine 5-deiodinase also known as type III iodothyronine deiodinase (EC number 1.21.99.3) is an enzyme that in humans is encoded by the DIO3 gene. This enzyme catalyses the following chemical reaction
The thyrotropin receptor is a receptor that responds to thyroid-stimulating hormone and stimulates the production of thyroxine (T4) and triiodothyronine (T3). The TSH receptor is a member of the G protein-coupled receptor superfamily of integral membrane proteins and is coupled to the Gs protein.

The hypothalamic–pituitary–thyroid axis is part of the neuroendocrine system responsible for the regulation of metabolism and also responds to stress.

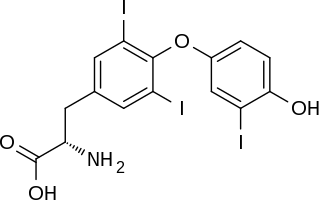
Reverse triiodothyronine (3,3′,5′-triiodothyronine, reverse T3, or rT3) is an isomer of triiodothyronine (3,5,3′ triiodothyronine, T3).
An antithyroid agent is a hormone inhibitor acting upon thyroid hormones.

The sodium/iodide cotransporter, also known as the sodium/iodide symporter (NIS), is a protein that in humans is encoded by the SLC5A5 gene. It is a transmembrane glycoprotein with a molecular weight of 87 kDa and 13 transmembrane domains, which transports two sodium cations (Na+) for each iodide anion (I−) into the cell. NIS mediated uptake of iodide into follicular cells of the thyroid gland is the first step in the synthesis of thyroid hormone.

Type II iodothyronine deiodinase is an enzyme that in humans is encoded by the DIO2 gene.

Thyroid hormones are any hormones produced and released by the thyroid gland, namely triiodothyronine (T3) and thyroxine (T4). They are tyrosine-based hormones that are primarily responsible for regulation of metabolism. T3 and T4 are partially composed of iodine, derived from food. A deficiency of iodine leads to decreased production of T3 and T4, enlarges the thyroid tissue and will cause the disease known as simple goitre.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Deiodinase (monodeiodinase) is a peroxidase enzyme that is involved in the activation or deactivation of thyroid hormones.

3-Iodotyrosine is an intermediate in the synthesis of thyroid hormones which is derived from iodination of tyrosine at the meta-position of the benzene ring. One unit can combine with diiodotyrosine to form triiodothyronine, as occurs in the colloid of the thyroid follicle. Two units can combine to form 3,3'-diiodothyronine.
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.

The Plummer effect is one of several physiological feedforward mechanisms taking place in follicular cells of the healthy thyroid gland and preventing the development of thyrotoxicosis in situations of extremely high supply with iodine.